文章

1235865-75-4,Venetoclax
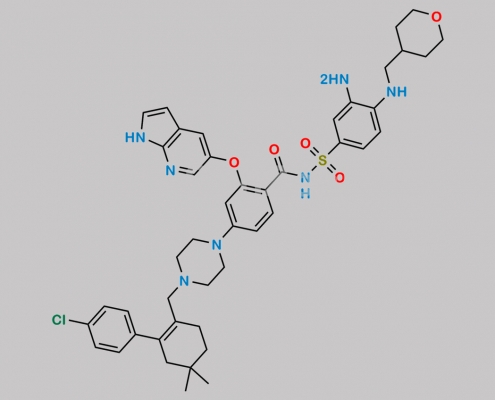
2247750-11-2,Venetoclax
Scroll to top

