文章
 https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-80.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
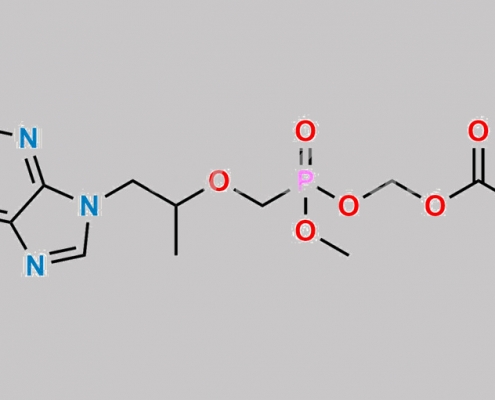
great_watson-int2024-07-08 16:27:322024-07-08 16:27:32Tenofovir 杂质 80 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-80.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 16:27:322024-07-08 16:27:32Tenofovir 杂质 80 CAS号 N/A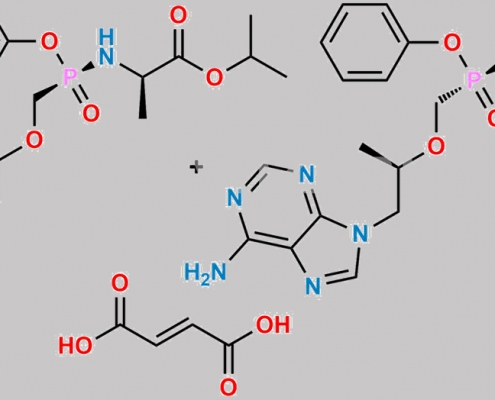 https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-59.jpg
524
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 16:06:332024-07-08 16:06:33Tenofovir 杂质 59 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-59.jpg
524
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 16:06:332024-07-08 16:06:33Tenofovir 杂质 59 CAS号 N/A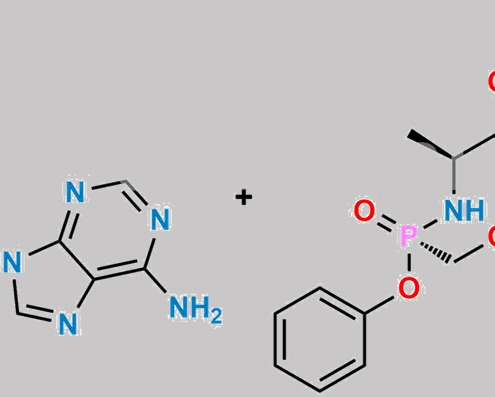 https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-61.jpg
397
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 16:06:332024-07-08 16:06:33Tenofovir 杂质 61 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-61.jpg
397
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 16:06:332024-07-08 16:06:33Tenofovir 杂质 61 CAS号 N/A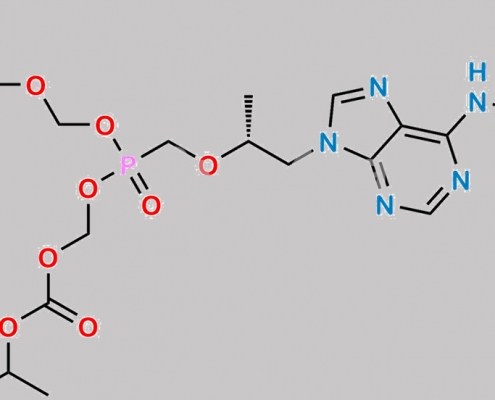
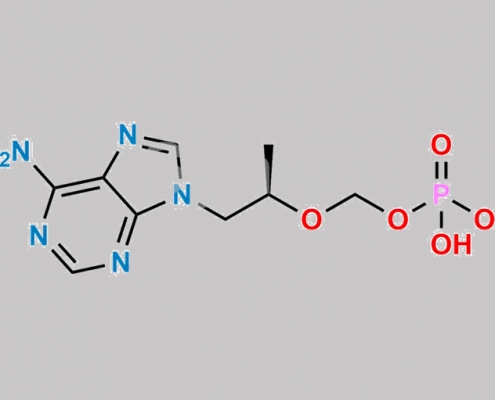
2514954-65-3,Tenofovir
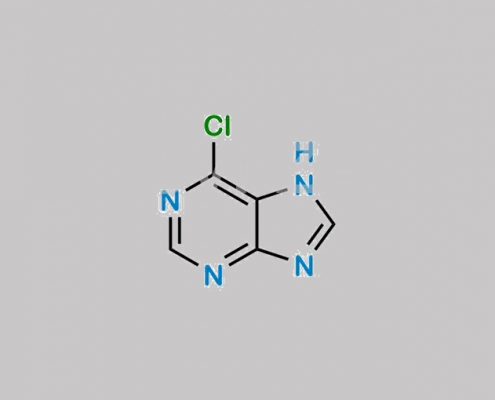
87-42-3,Tenofovir
 https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-71.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 14:37:522024-07-08 14:37:52Tenofovir 杂质 71 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-71.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 14:37:522024-07-08 14:37:52Tenofovir 杂质 71 CAS号 N/A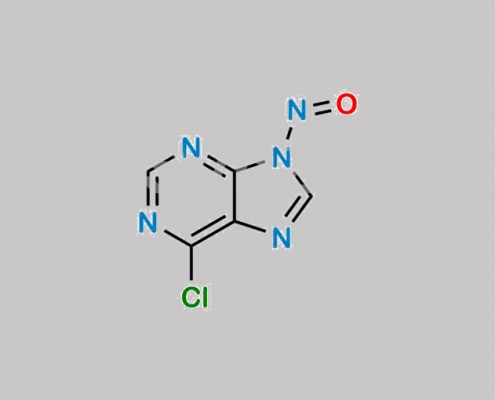 https://www.watson-int.cn/wp-content/uploads/2024/07/n-nitroso-tenofovir-impurity-1.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 14:36:222024-07-08 14:36:22N-Nitroso Tenofovir 杂质 1 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/n-nitroso-tenofovir-impurity-1.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 14:36:222024-07-08 14:36:22N-Nitroso Tenofovir 杂质 1 CAS号 N/A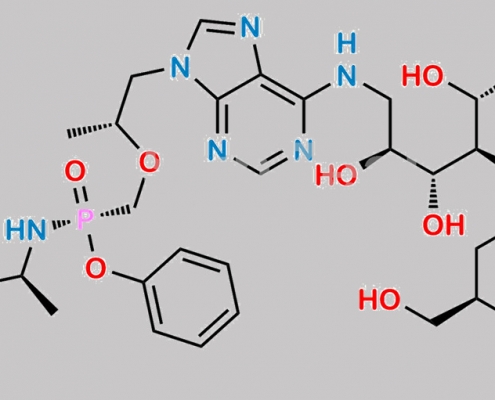 https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-83.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 14:36:222024-07-08 14:36:22Tenofovir 杂质 83 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/tenofovir-impurity-83.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 14:36:222024-07-08 14:36:22Tenofovir 杂质 83 CAS号 N/A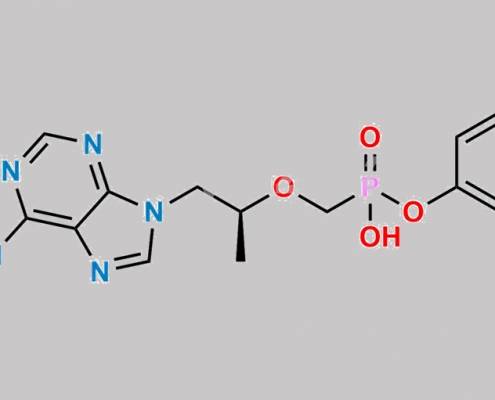
2488598-61-2,Tenofovir
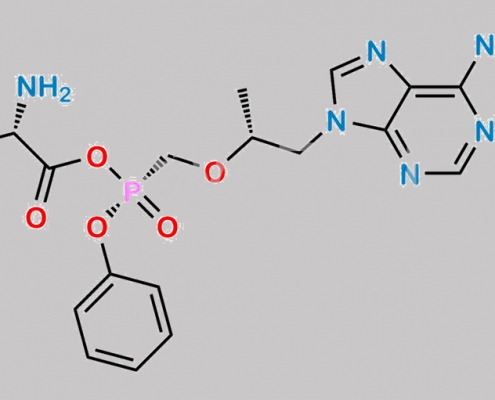
2228092-46-2,Tenofovir
Scroll to top















