文章
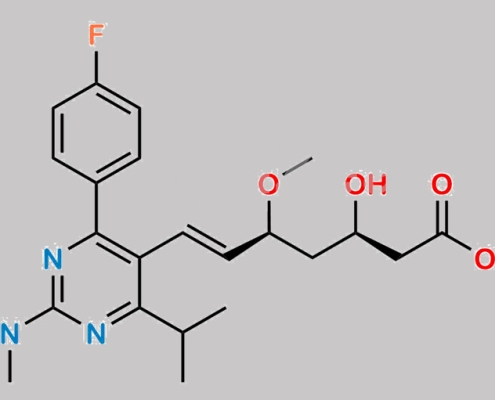
Rosuvastatin 杂质 76 CAS号 N/A
N/A,Rosuvastatin
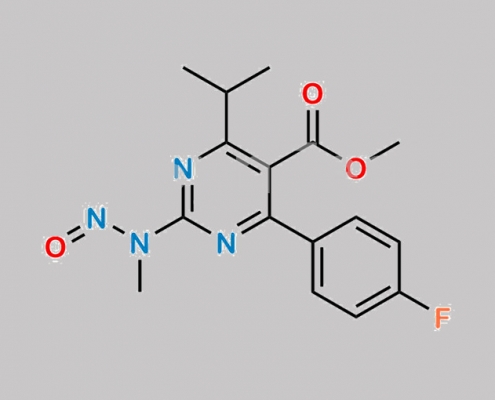
Rosuvastatin Nitroso 杂质 1 CAS号 N/A
N/A,Rosuvastatin

Rosuvastatin EP 杂质 K (Sodium salt) CAS号 N/A
N/A,Rosuvastatin

Rosuvastatin EP 杂质 K (Hemicalcium salt) CAS号 N/A
N/A,Rosuvastatin

Rosuvastatin EP 杂质 G (Sodium salt) CAS号 147098-18-8
147098-18-8,Rosuvastatin
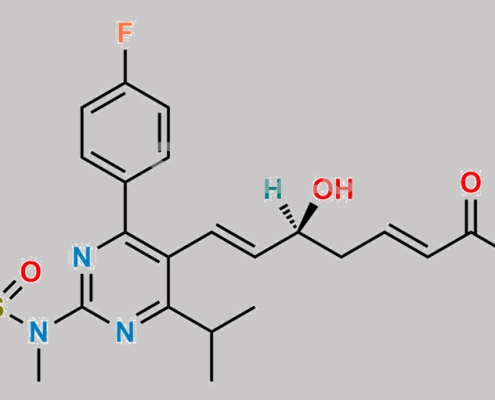
Rosuvastatin EP 杂质 N (Sodium salt) CAS号 N/A
N/A,Rosuvastatin

Rosuvastatin 杂质 59 CAS号 N/A
N/A,Rosuvastatin

Rosuvastatin 杂质 23 CAS号 N/A
N/A,Rosuvastatin

Rosuvastatin 杂质 56 CAS号 N/A
N/A,Rosuvastatin
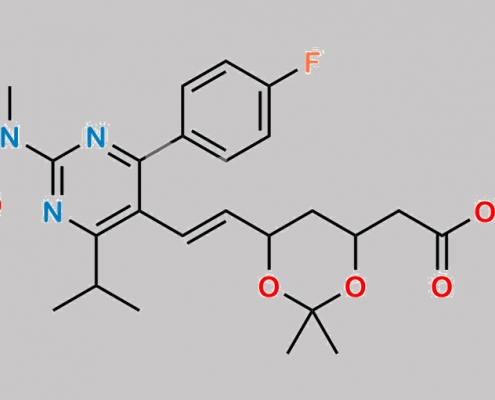
Rosuvastatin 杂质 69 CAS号 1007871-85-3
1007871-85-3,Rosuvastatin





