文章
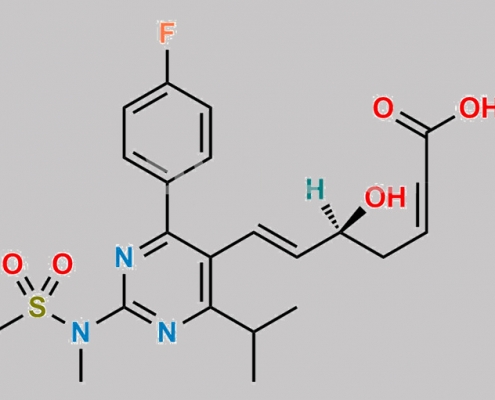
Rosuvastatin EP Impurity K CAS号 1422954-12-8
1422954-12-8,Rosuvastatin
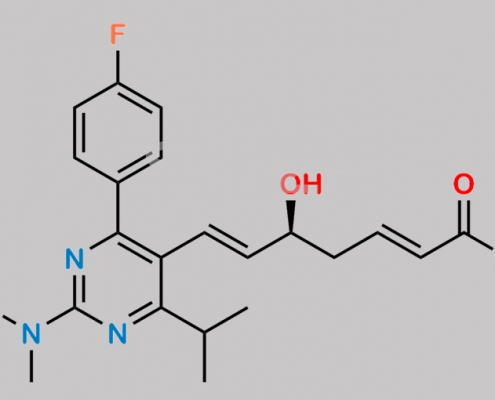
Rosuvastatin 2,3-Anhydro Acid Methyl Ester CAS号 2452383-22-9
2452383-22-9,Rosuvastatin
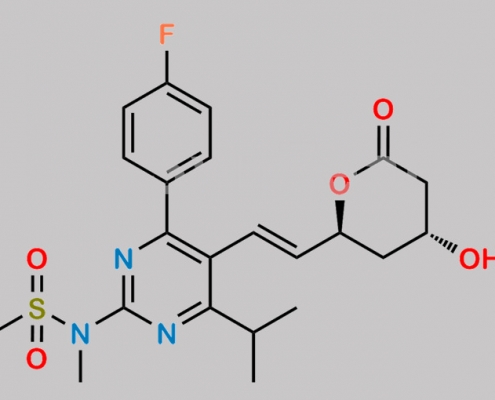
Rosuvastatin EP Impurity D CAS号 503610-43-3
503610-43-3,Rosuvastatin
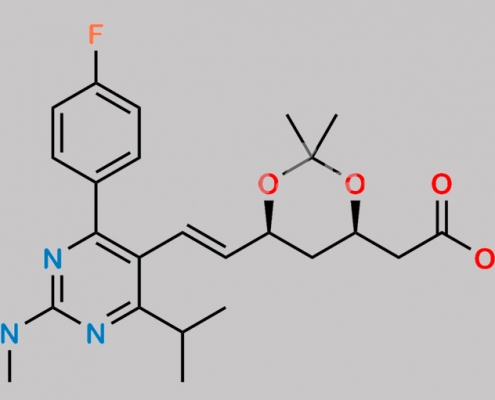
Rosuvastatin EP Impurity F CAS号 289042-12-2
289042-12-2,Rosuvastatin
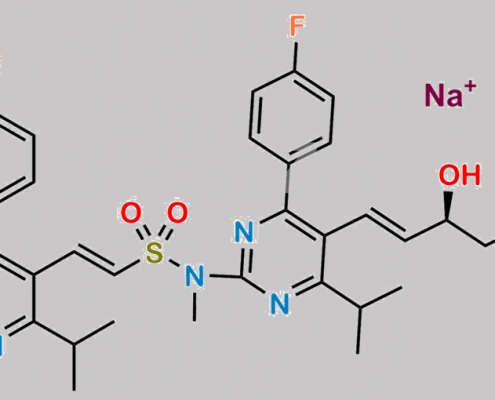
Rosuvastatin EP Impurity J (Sodium Salt) CAS号 N/A
N/A,Rosuvastatin
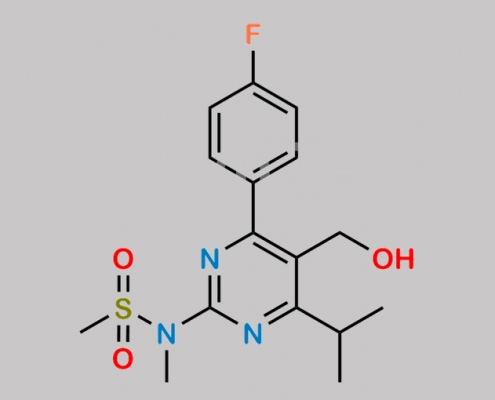
Rosuvastatin Hydroxymethyl Impurity CAS号 147118-36-3
147118-36-3,Rosuvastatin
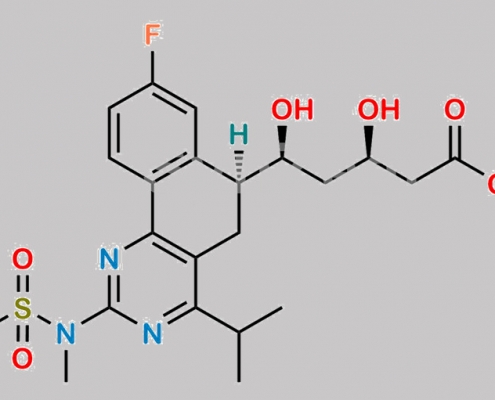
Rosuvastatin (6S)-Isomer Hemicalcium Salt CAS号 854898-50-3
854898-50-3,Rosuvastatin
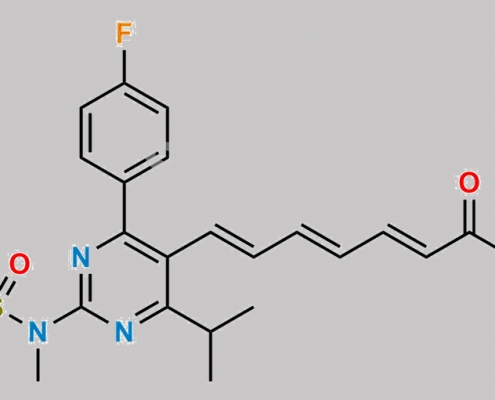
Rosuvastatin 2,3,4,5-Dianhydro Acid CAS号 N/A
N/A,Rosuvastatin
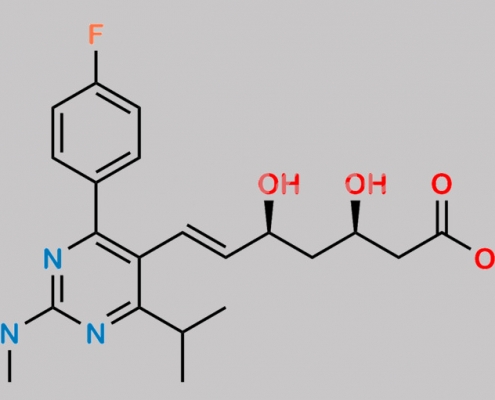
Rosuvastatin Acid t-Butyl Ester CAS号 355806-00-7
355806-00-7,Rosuvastatin
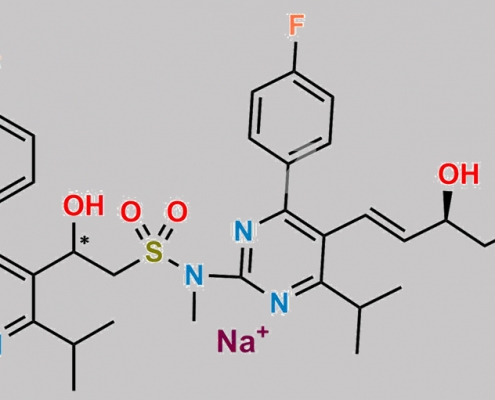
Rosuvastatin EP Impurity E (Sodium Salt) CAS号 N/A
N/A,Rosuvastatin





