文章
1257527-98-2,Rivaroxaban
 https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-di-thiphene-impurity.jpg
624
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 22:39:042024-07-08 22:39:04Rivaroxaban Di-thiphene 杂质 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-di-thiphene-impurity.jpg
624
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 22:39:042024-07-08 22:39:04Rivaroxaban Di-thiphene 杂质 CAS号 N/A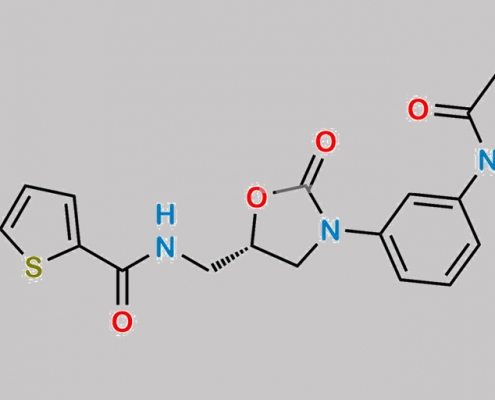 https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-impurity-110.jpg
512
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 22:34:432024-07-08 22:34:43Rivaroxaban 杂质 110 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-impurity-110.jpg
512
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 22:34:432024-07-08 22:34:43Rivaroxaban 杂质 110 CAS号 N/A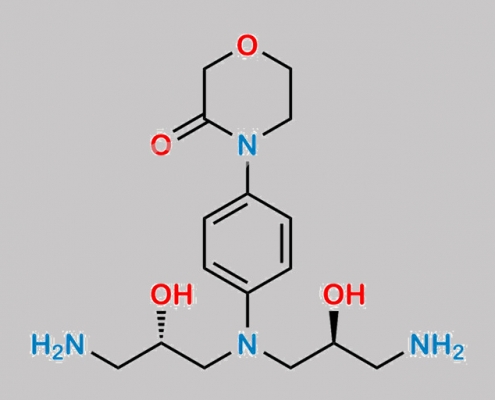 https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-impurity-112.jpg
512
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 22:34:432024-07-08 22:34:43Rivaroxaban 杂质 112 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-impurity-112.jpg
512
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 22:34:432024-07-08 22:34:43Rivaroxaban 杂质 112 CAS号 N/A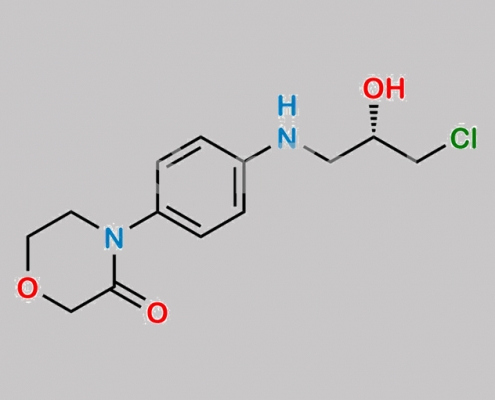
1252018-21-5,Rivaroxaban
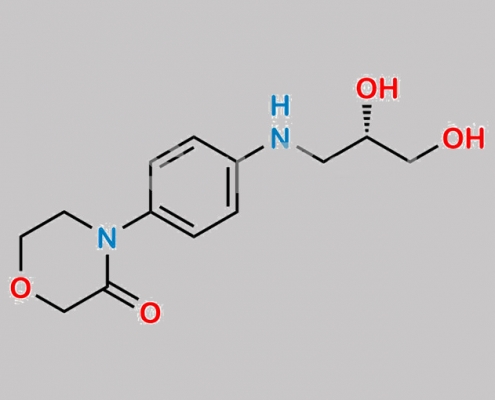
2733521-54-3,Rivaroxaban
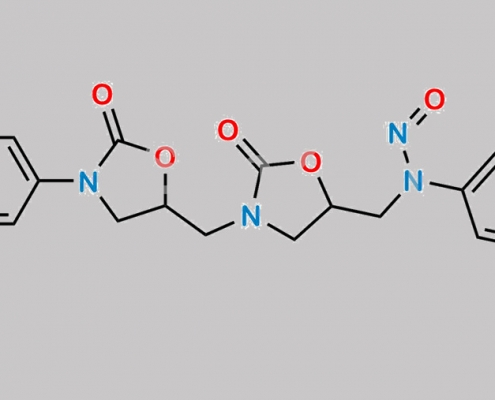 https://www.watson-int.cn/wp-content/uploads/2024/07/n-nitroso-rivaroxaban-dioxazolidine-impurity.jpg
507
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 21:17:362024-07-08 21:17:36N-Nitroso Rivaroxaban Dioxazolidine 杂质 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/n-nitroso-rivaroxaban-dioxazolidine-impurity.jpg
507
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 21:17:362024-07-08 21:17:36N-Nitroso Rivaroxaban Dioxazolidine 杂质 CAS号 N/A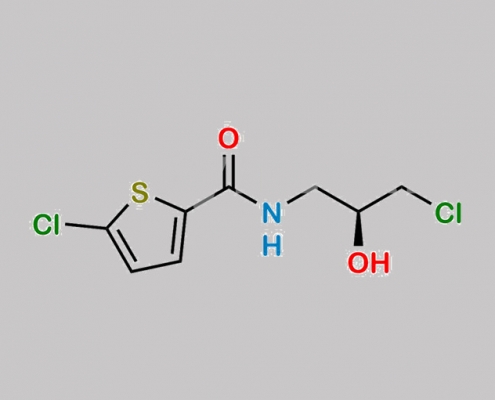
1384257-81-1,Rivaroxaban
 https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-nitroso-impurity-12.jpg
544
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 20:39:042024-07-08 20:39:04Rivaroxaban Nitroso 杂质 12 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-nitroso-impurity-12.jpg
544
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
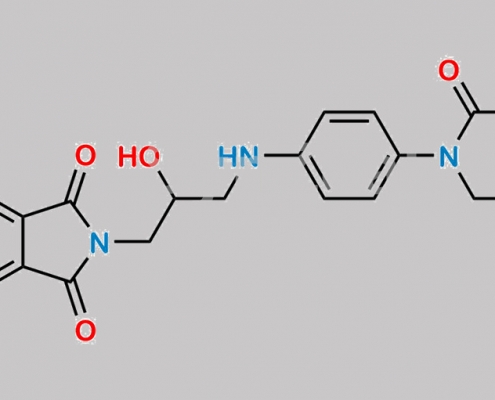
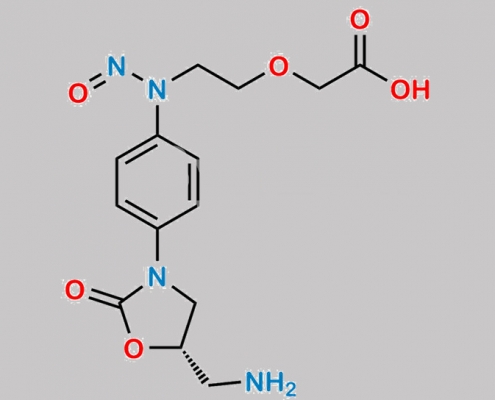
great_watson-int2024-07-08 20:39:042024-07-08 20:39:04Rivaroxaban Nitroso 杂质 12 CAS号 N/A https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-amino-acid-phthalimide-nitroso-impurity.jpg
655
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 20:39:042024-07-08 20:39:04Rivaroxaban Amino Acid Phthalimide Nitroso 杂质 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/rivaroxaban-amino-acid-phthalimide-nitroso-impurity.jpg
655
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 20:39:042024-07-08 20:39:04Rivaroxaban Amino Acid Phthalimide Nitroso 杂质 CAS号 N/A
Scroll to top















