文章
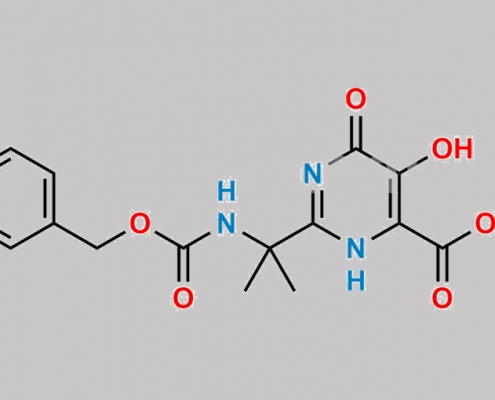
519032-08-7,Raltegravir
 https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-impurity-14.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 22:47:542024-07-08 22:47:54Raltegravir 杂质 14 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-impurity-14.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
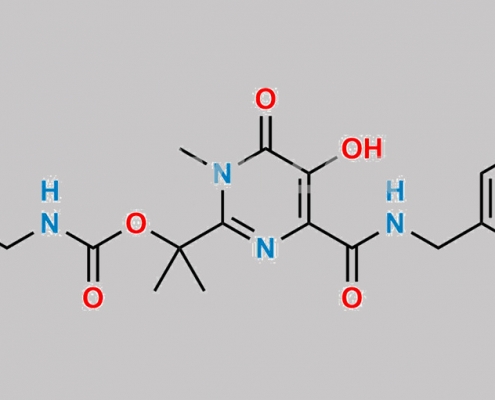
great_watson-int2024-07-08 22:47:542024-07-08 22:47:54Raltegravir 杂质 14 CAS号 N/A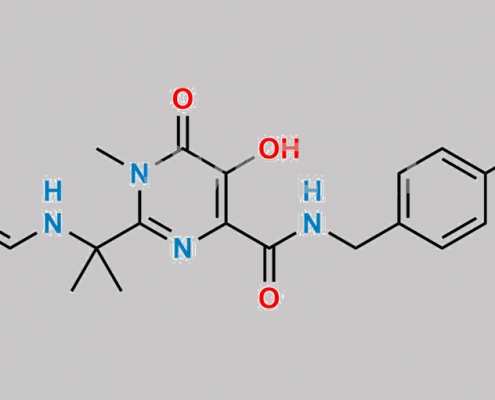 https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-formyl-impurity.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 22:19:062024-07-08 22:19:06Raltegravir Formyl 杂质 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-formyl-impurity.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 22:19:062024-07-08 22:19:06Raltegravir Formyl 杂质 CAS号 N/A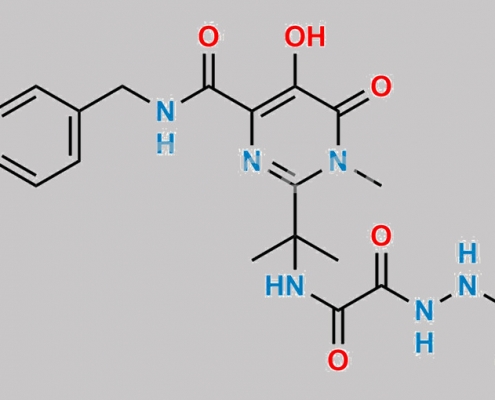
2525279-50-7,Raltegravir
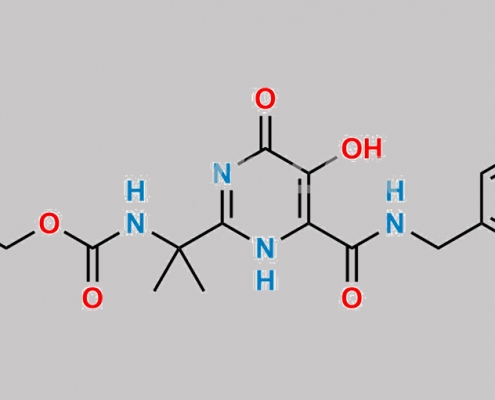
519028-33-2,Raltegravir
 https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-impurity-5.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 19:35:582024-07-08 19:35:58Raltegravir 杂质 5 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-impurity-5.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 19:35:582024-07-08 19:35:58Raltegravir 杂质 5 CAS号 N/A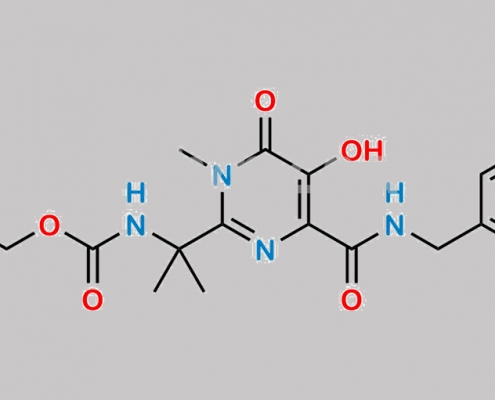
518048-02-7,Raltegravir
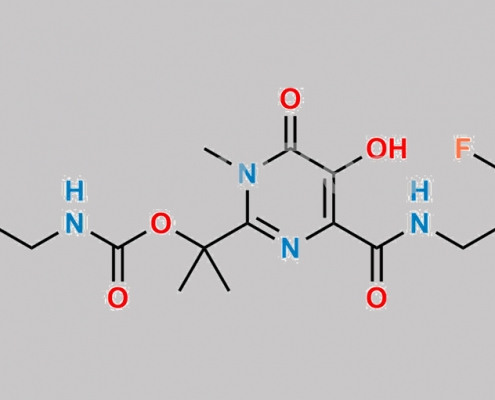 https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-impurity-13.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 18:02:572024-07-08 18:02:57Raltegravir 杂质 13 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-impurity-13.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 18:02:572024-07-08 18:02:57Raltegravir 杂质 13 CAS号 N/A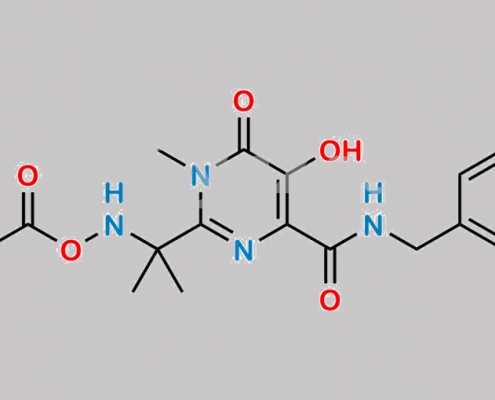 https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-impurity-4.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 17:48:332024-07-08 17:48:33Raltegravir 杂质 4 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/raltegravir-impurity-4.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 17:48:332024-07-08 17:48:33Raltegravir 杂质 4 CAS号 N/A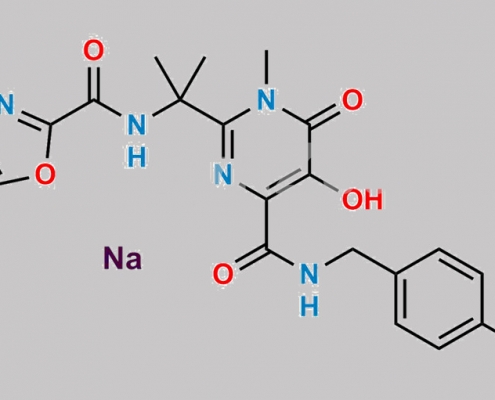
1292804-07-9,Raltegravir
Scroll to top















