标签存档: Prednicarbate
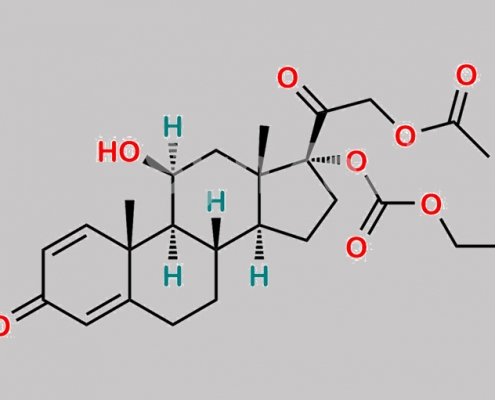
Prednicarbate EP 杂质 E CAS号 671225-23-3
671225-23-3,Prednicarbate
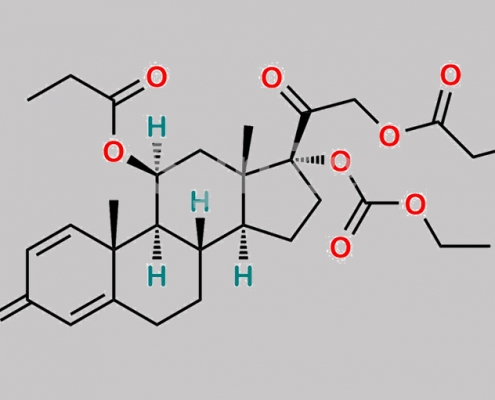
Prednicarbate EP 杂质 G CAS号 N/A
N/A,Prednicarbate
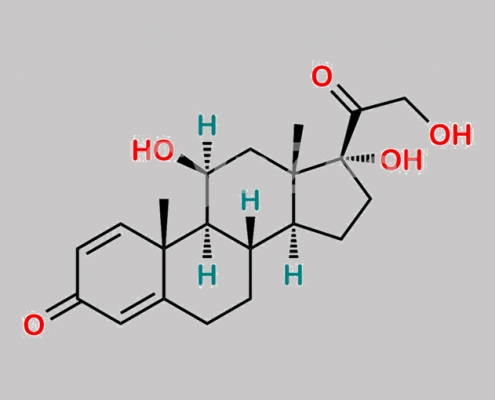
Prednicarbate EP 杂质 A CAS号 50-24-8
50-24-8,Prednicarbate
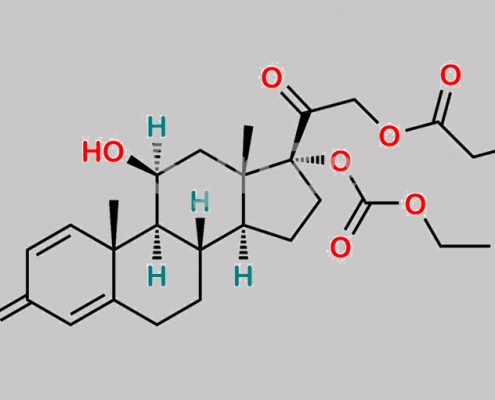
Prednicarbate CAS号 73771-04-7
73771-04-7,Prednicarbate
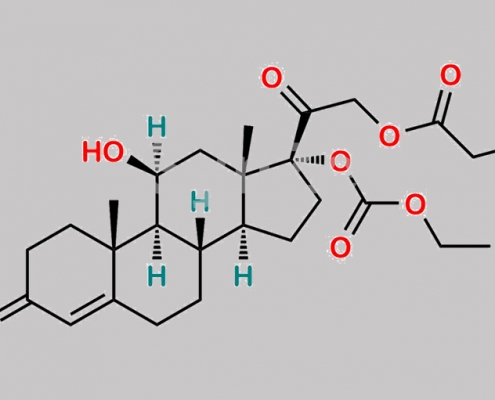
Prednicarbate EP 杂质 F CAS号 671225-26-6
671225-26-6,Prednicarbate

Prednicarbate EP 杂质 C CAS号 5740-62-5
5740-62-5,Prednicarbate
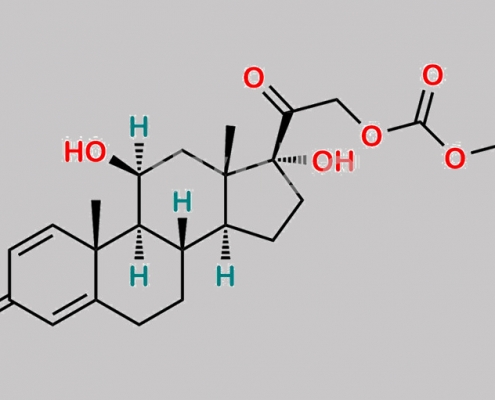
Prednicarbate EP 杂质 D CAS号 2205-88-1
2205-88-1,Prednicarbate
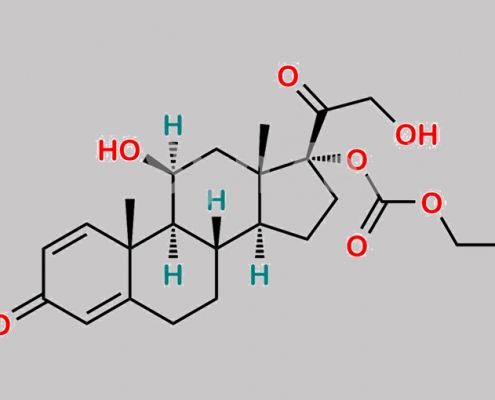
Prednicarbate EP 杂质 B CAS号 104286-02-4
104286-02-4,Prednicarbate





