文章
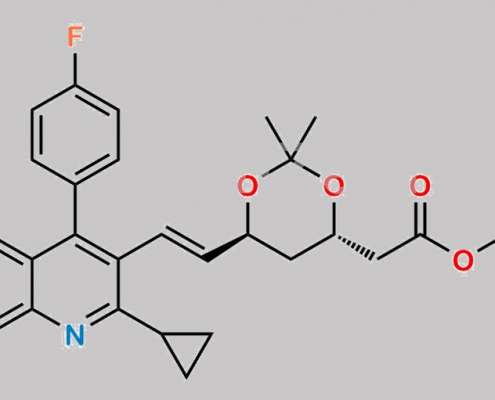
Pitavastatin Impurity 6 CAS号 2347430-68-4
2347430-68-4,Pitavastatin
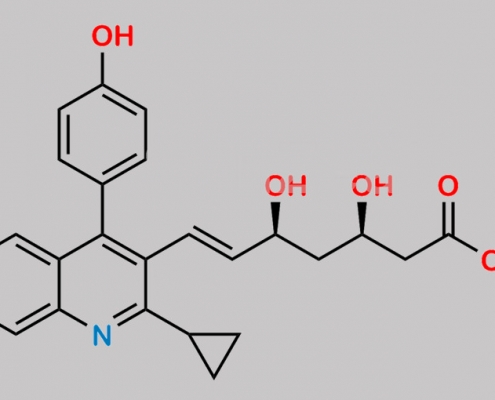
Pitavastatin Impurity 13 CAS号 847849-72-3
847849-72-3,Pitavastatin
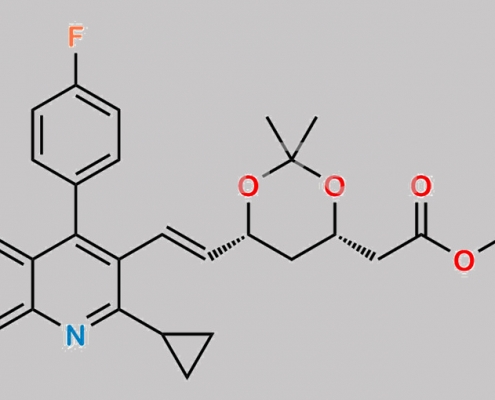
Pitavastatin Impurity 5 CAS号 167934-29-4
167934-29-4,Pitavastatin
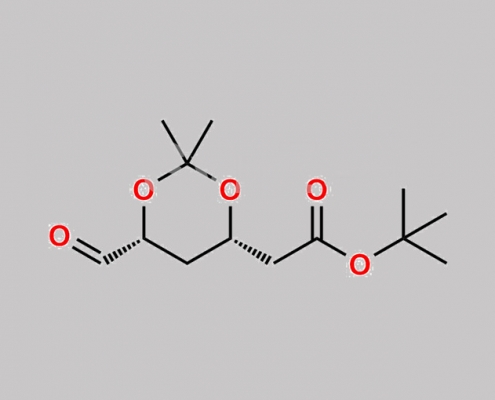
Pitavastatin Impurity 7 CAS号 1044518-75-3
1044518-75-3,Pitavastatin
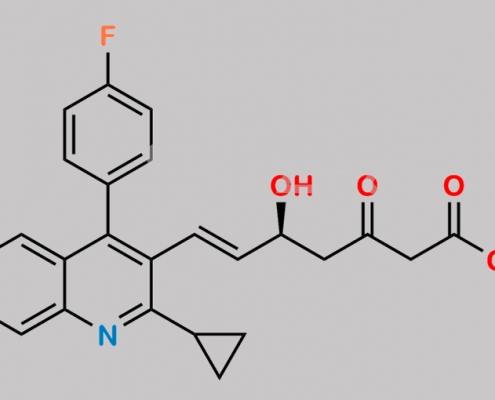
Pitavastatin 3-Oxo Sodium CAS号 N/A
N/A,Pitavastatin
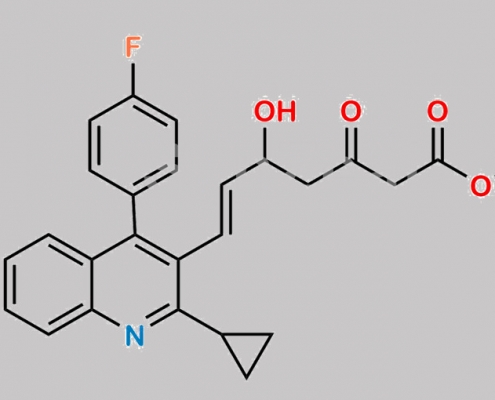
Pitavastatin 3-Oxo Ethyl Ester CAS号 148901-69-3
148901-69-3,Pitavastatin
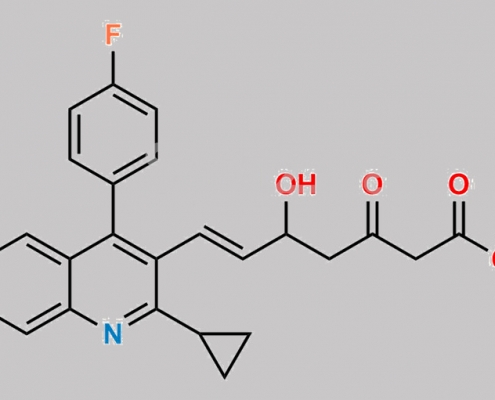
Pitavastatin-3-Oxo Sodium Salt CAS号 N/A
N/A,Pitavastatin
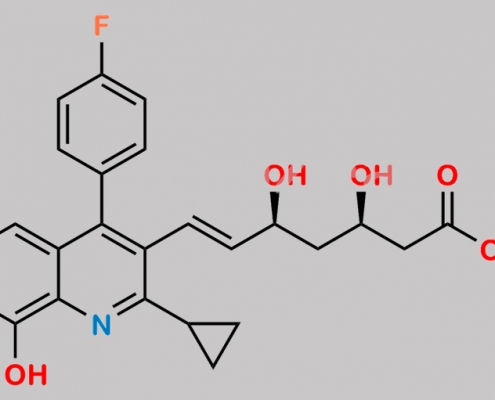
Pitavastatin 8-Hydroxy Impurity CAS号 224320-09-6
224320-09-6,Pitavastatin





