文章
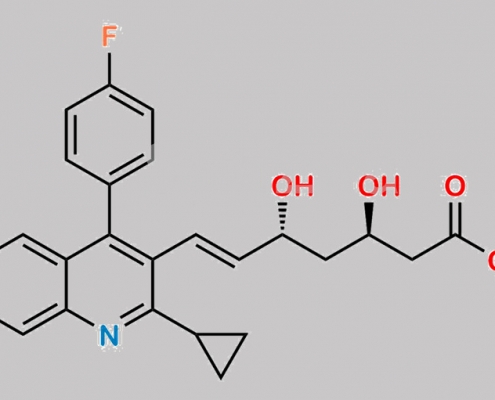
Pitavastatin (3R,5R)-Isomer Sodium Salt CAS号 N/A
N/A,Pitavastatin
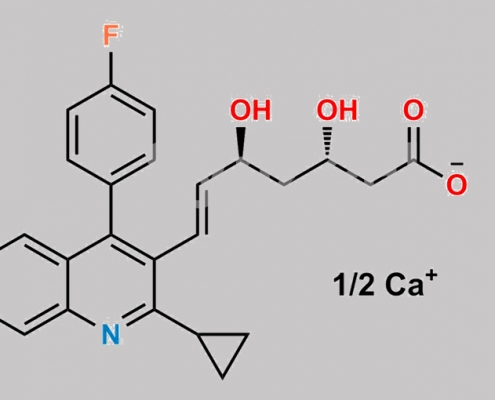
Pitavastatin (3S,5S)-Isomer Calcium CAS号 254452-92-1
254452-92-1,Pitavastatin
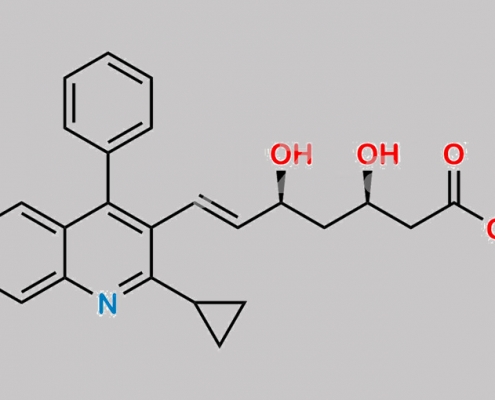
Pitavastatin Desfluoro Impurity (Calcium Salt) CAS号 1258947-30-6
1258947-30-6,Pitavastatin
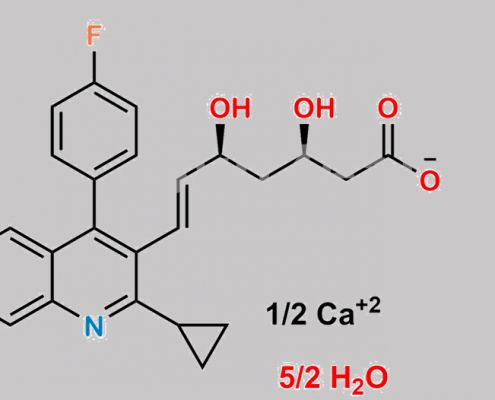
Pitavastatin Calcium Pentahydrate CAS号 1852536-33-4
1852536-33-4,Pitavastatin
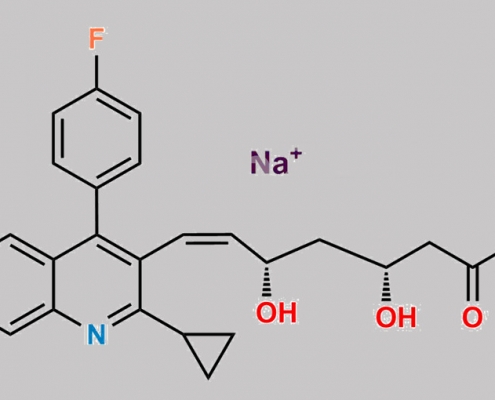
Pitavastatin Sodium (Z)-Isomer CAS号 1633767-50-6
1633767-50-6,Pitavastatin
 https://www.watson-int.cn/wp-content/uploads/2024/07/pitavastatin-dihydrobenzophenanthridine-impurity-na-salt.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
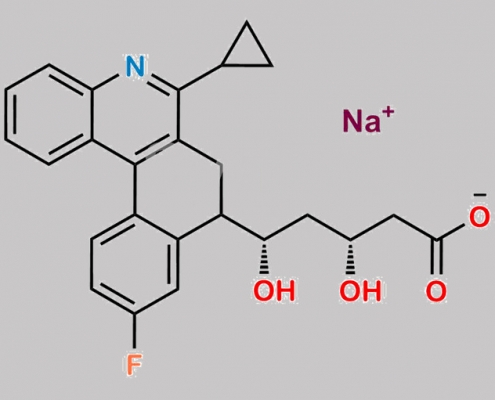
great_watson-int2024-07-07 00:37:132024-07-07 00:37:13Pitavastatin Dihydrobenzophenanthridine Impurity (Na salt) CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/pitavastatin-dihydrobenzophenanthridine-impurity-na-salt.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-07 00:37:132024-07-07 00:37:13Pitavastatin Dihydrobenzophenanthridine Impurity (Na salt) CAS号 N/A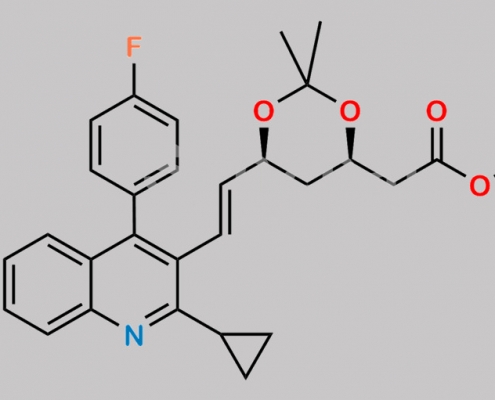
Pitavastatin Acetonide t-Butyl Ester CAS号 147489-06-3
147489-06-3,Pitavastatin
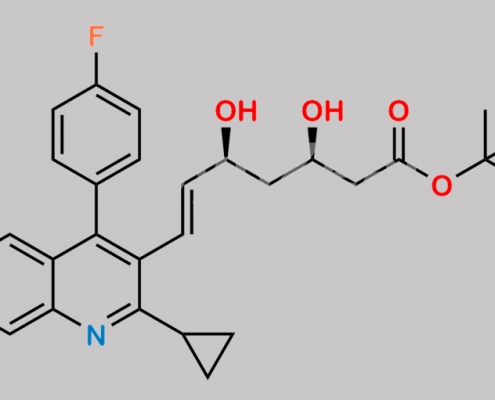
Pitavastatin t-Butyl Ester CAS号 586966-54-3
586966-54-3,Pitavastatin
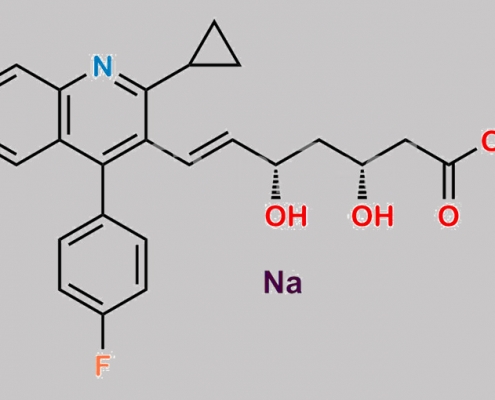
Pitavastatin Sodium CAS号 574705-92-3
574705-92-3,Pitavastatin
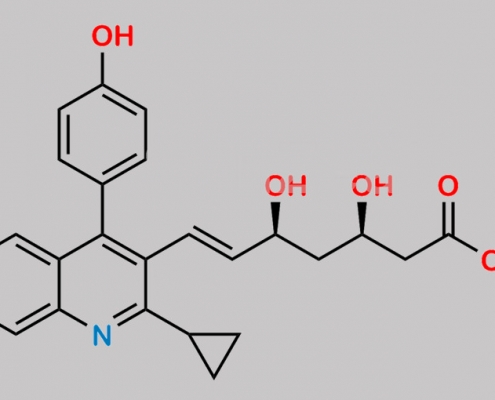
Pitavastatin Impurity 13 CAS号 847849-72-3
847849-72-3,Pitavastatin





