文章
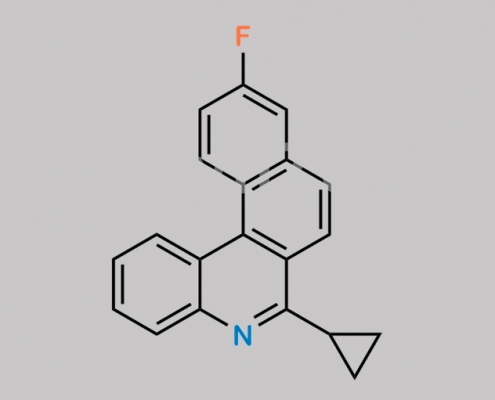
Pitavastatin 杂质 4 CAS号 1187966-95-5
1187966-95-5,Pitavastatin
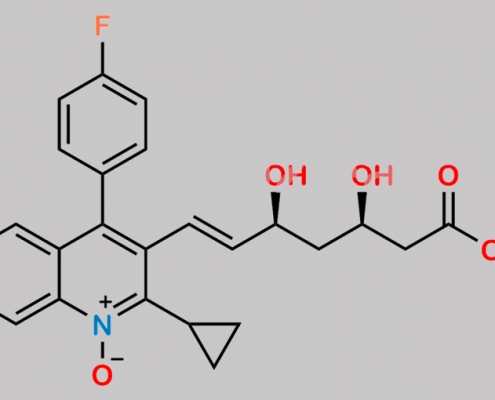
Pitavastatin 杂质 15 CAS号 1611499-16-1
1611499-16-1,Pitavastatin
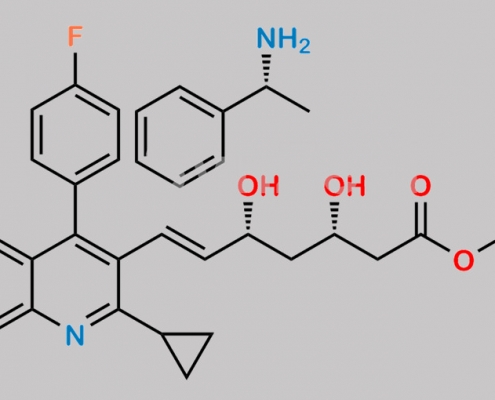
Pitavastatin 杂质 9 CAS号 176593-07-0
176593-07-0,Pitavastatin
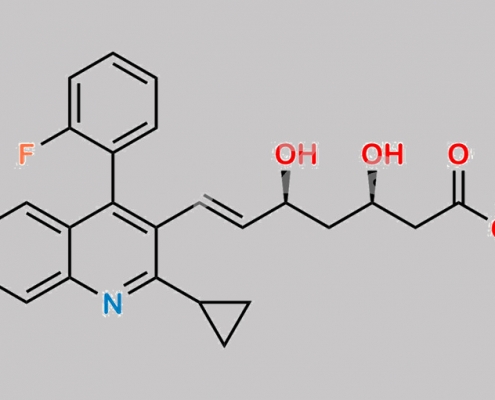
Pitavastatin 杂质 11 CAS号 N/A
N/A,Pitavastatin

Pitavastatin Methyl Ester CAS号 849811-78-5
849811-78-5,Pitavastatin
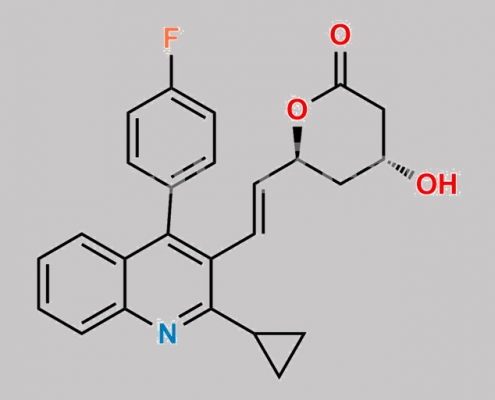
Pitavastatin Lactone CAS号 141750-63-2
141750-63-2,Pitavastatin
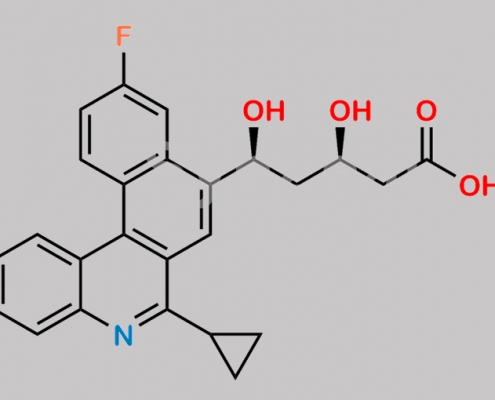
Pitavastatin Benzophenanthridine 杂质 CAS号 1187966-93-3
1187966-93-3,Pitavastatin
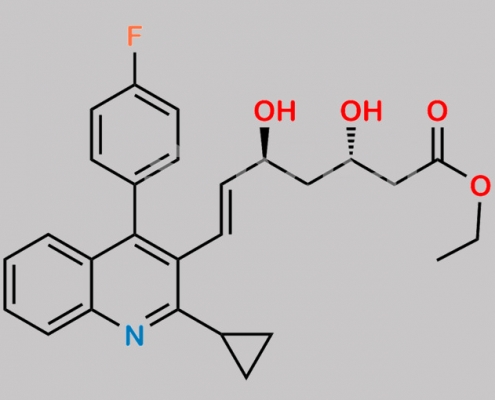
Pitavastatin (3S,5S)-Isomer Ethyl Ester CAS号 380848-30-6
380848-30-6,Pitavastatin
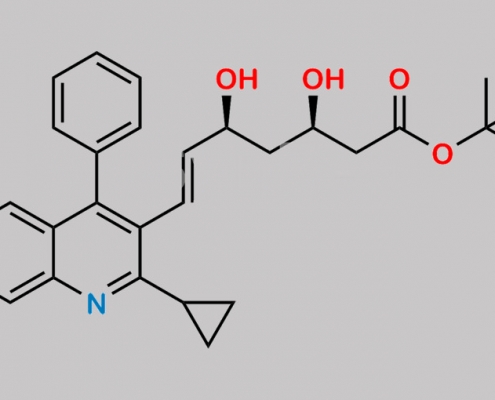
Pitavastatin Desfluoro t-Butyl Ester CAS号 N/A
N/A,Pitavastatin
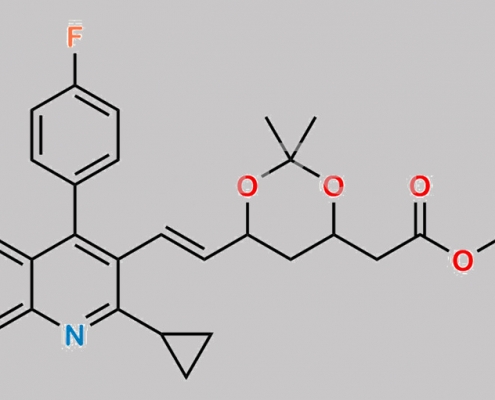
Pitavastatin 杂质 16 CAS号 160495-72-7
160495-72-7,Pitavastatin





