文章 2024年7月8日
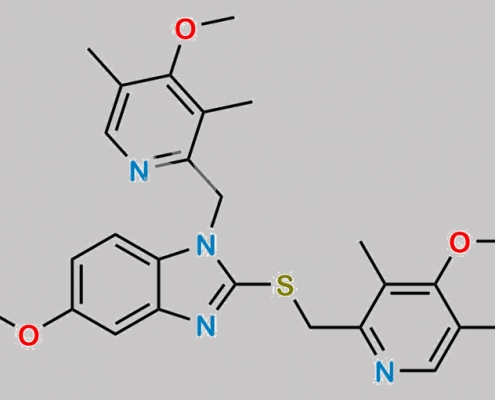
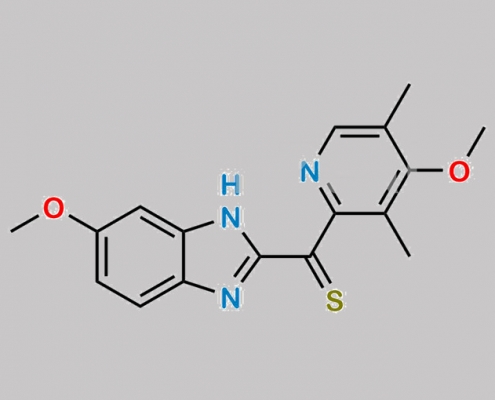
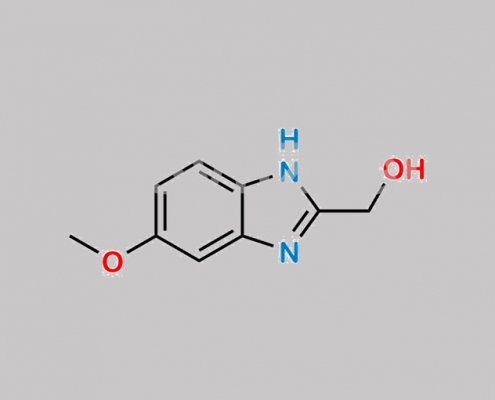
1346600-29-0,Omeprazole
https://www.watson-int.cn/wp-content/uploads/2024/07/omeprazole-impurity-30.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 18:04:27 2024-07-08 18:04:27 Omeprazole 杂质 30 CAS号 1346600-29-0 2024年7月8日
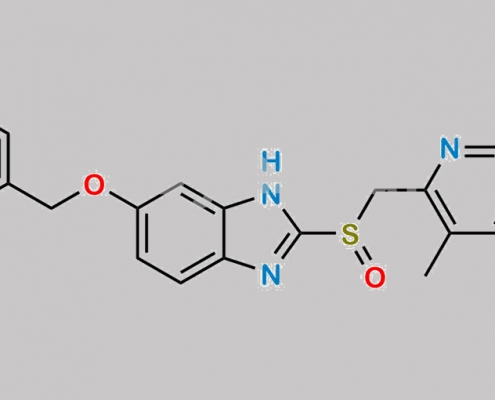
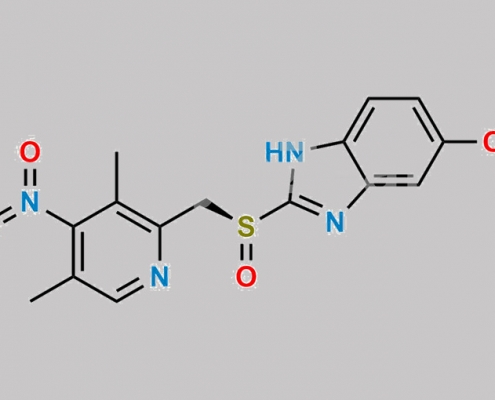
1215799-39-5,Omeprazole
https://www.watson-int.cn/wp-content/uploads/2024/07/5-benzyloxy-omeprazole.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 16:23:45 2024-07-08 16:23:45 5-Benzyloxy Omeprazole CAS号 1215799-39-5 2024年7月8日
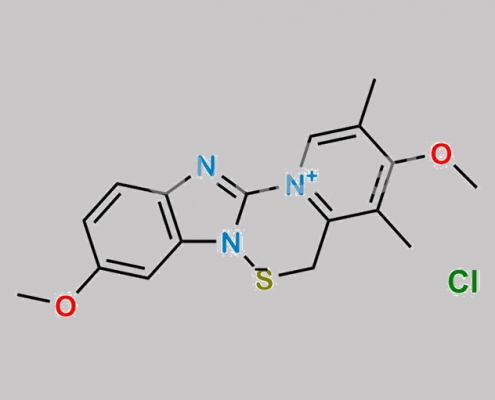
477585-18-5,Omeprazole
https://www.watson-int.cn/wp-content/uploads/2024/07/omeprazole-impurity-35.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 16:23:21 2024-07-08 16:23:21 Omeprazole 杂质 35 CAS号 477585-18-5 2024年7月8日
121459-89-0,Omeprazole
https://www.watson-int.cn/wp-content/uploads/2024/07/omeprazole-impurity-18.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 16:20:33 2024-07-08 16:20:33 Omeprazole 杂质 18 CAS号 121459-89-0
https://www.watson-int.cn/wp-content/uploads/2024/07/omeprazole-impurity-34.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 16:12:16 2024-07-08 16:12:16 Omeprazole 杂质 34 CAS号 N/A 2024年7月8日
853950-49-9,Omeprazole
https://www.watson-int.cn/wp-content/uploads/2024/07/omeprazole-impurity-42.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 16:00:39 2024-07-08 16:00:39 Omeprazole 杂质 42 CAS号 853950-49-9
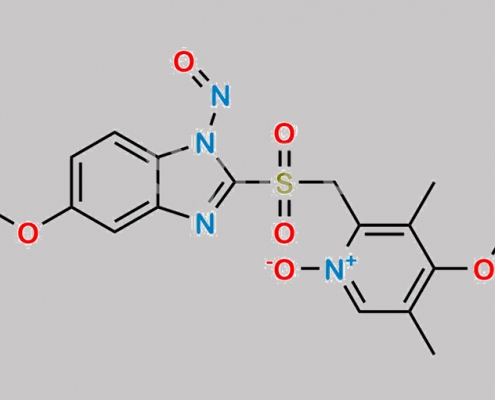
https://www.watson-int.cn/wp-content/uploads/2024/07/n-nitroso-omeprazole-ep-impurity-i.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 15:51:57 2024-07-08 15:51:57 N-Nitroso Omeprazole EP 杂质 I CAS号 N/A 2024年7月8日
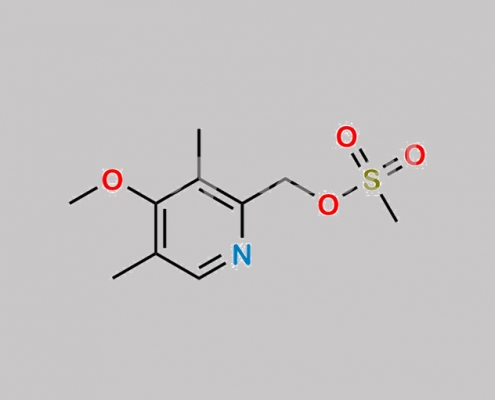
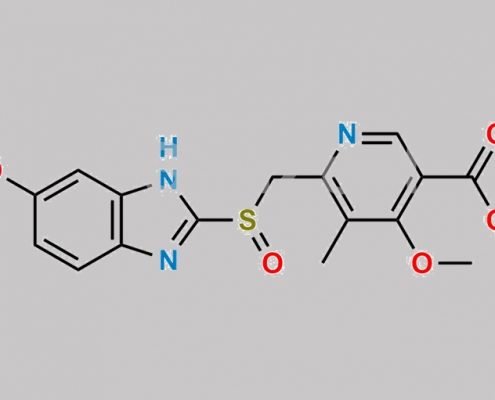
120003-83-0,Omeprazole
https://www.watson-int.cn/wp-content/uploads/2024/07/omeprazole-acid-methyl-ester.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 15:42:27 2024-07-08 15:42:27 Omeprazole Acid Methyl Ester CAS号 120003-83-0 2024年7月8日
20033-99-2,Omeprazole
https://www.watson-int.cn/wp-content/uploads/2024/07/omeprazole-impurity-29.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 15:35:36 2024-07-08 15:35:36 Omeprazole 杂质 29 CAS号 20033-99-2 2024年7月8日
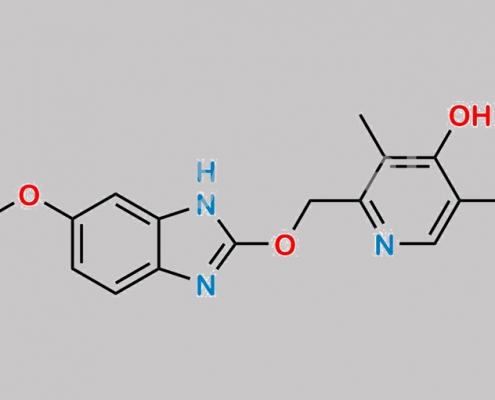
2451927-95-8,Omeprazole
https://www.watson-int.cn/wp-content/uploads/2024/07/omeprazole-impurity-27.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-08 14:36:22 2024-07-08 14:36:22 Omeprazole 杂质 27 CAS号 2451927-95-8
Scroll to top