文章
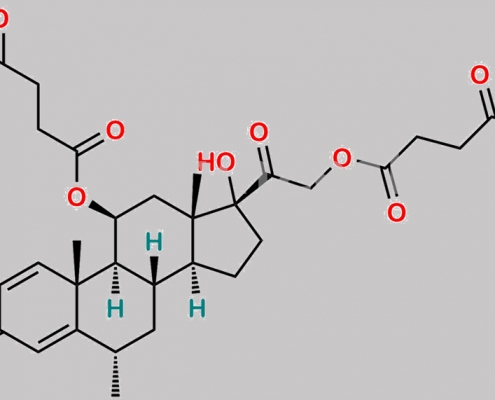
Methylprednisolone 杂质 16 CAS号 N/A
N/A,Methylprednisolone
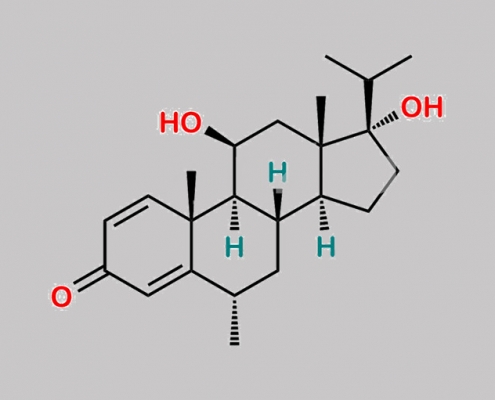
Methylprednisolone 杂质 11 CAS号 N/A
N/A,Methylprednisolone
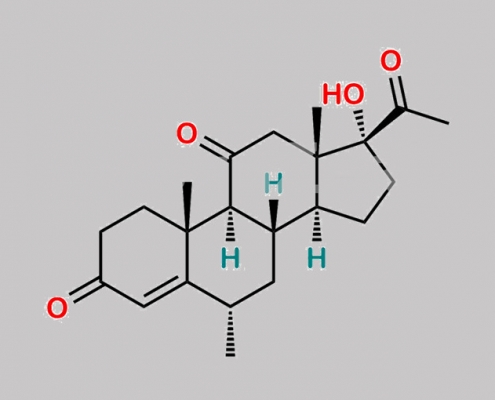
Methylprednisolone 杂质 14 CAS号 82332-00-1
82332-00-1,Methylprednisolone
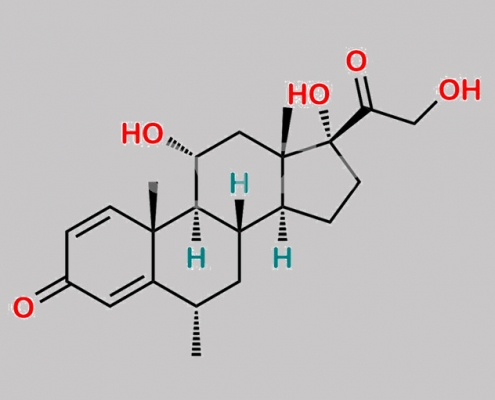
Methylprednisolone 杂质 17 CAS号 105500-05-8
105500-05-8,Methylprednisolone
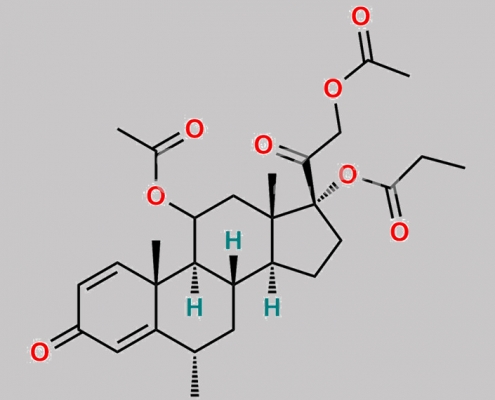
Methylprednisolone-17-propionate-11, 21 diacetate CAS号 N/A
N/A,Methylprednisolone
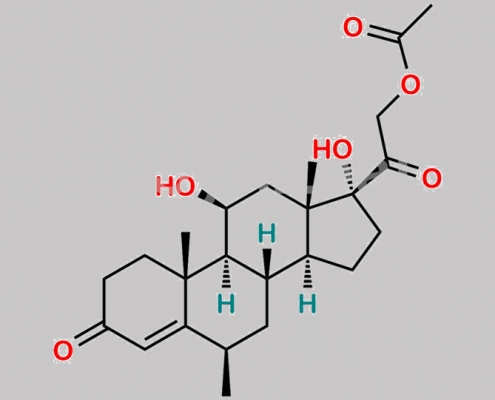
Methylprednisolone 杂质 10 CAS号 115223-47-7
115223-47-7,Methylprednisolone
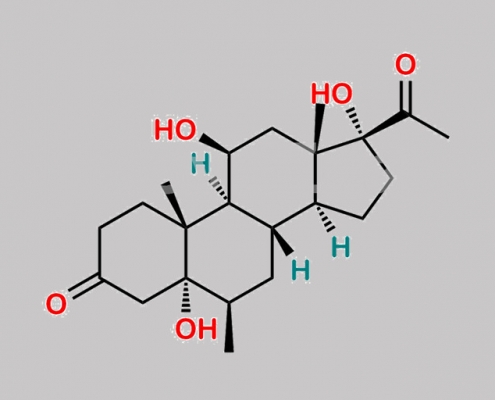
Methylprednisolone 杂质 12 CAS号 113251-88-0
113251-88-0,Methylprednisolone
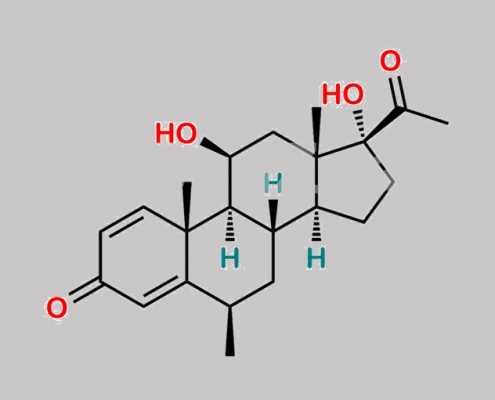
Methylprednisolone 杂质 13 CAS号 1048031-80-6
1048031-80-6,Methylprednisolone
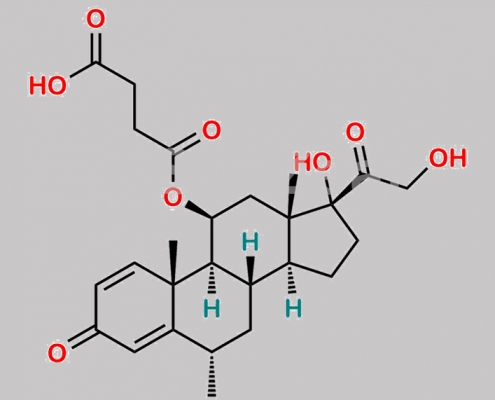
Methylprednisolone 杂质 15 CAS号 N/A
N/A,Methylprednisolone
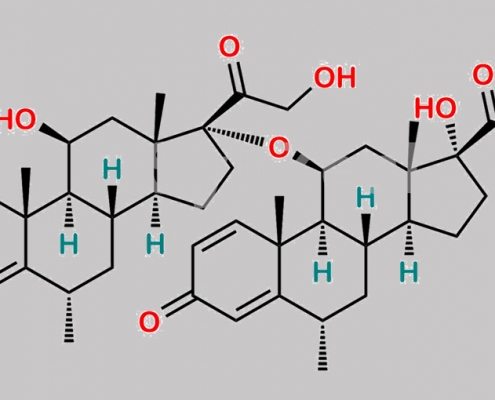
Methylprednisolone Dimer 杂质 (RRT 2.28) CAS号 N/A
N/A,Methylprednisolone





