文章
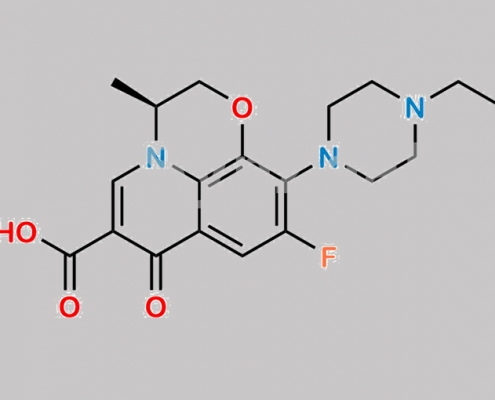
N-Ethyl Levofloxacin CAS号 106939-30-4
106939-30-4,Levofloxacin
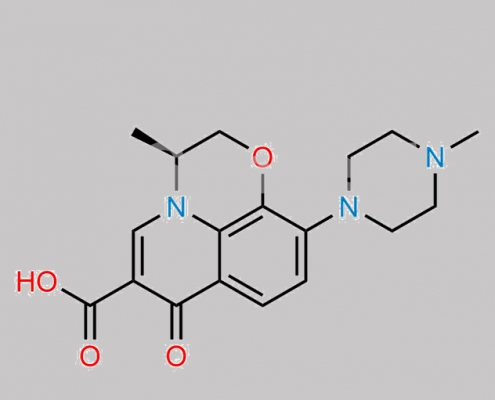
Levofloxacin EP 杂质 D CAS号 117620-85-6
117620-85-6,Levofloxacin
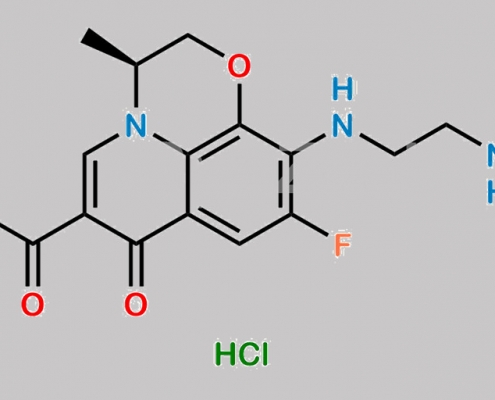
Levofloxacin EP 杂质 G (盐酸盐) CAS号 1346603-62-0
1346603-62-0,Levofloxacin
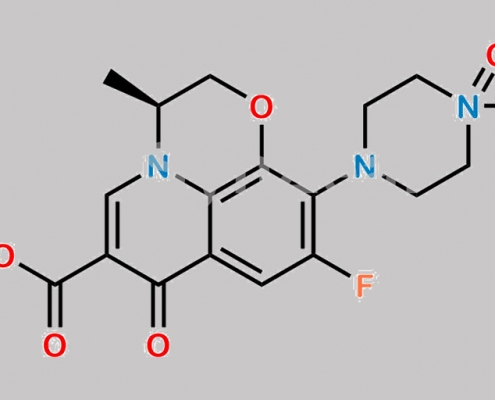
Levofloxacin EP 杂质 C CAS号 117678-38-3
117678-38-3,Levofloxacin
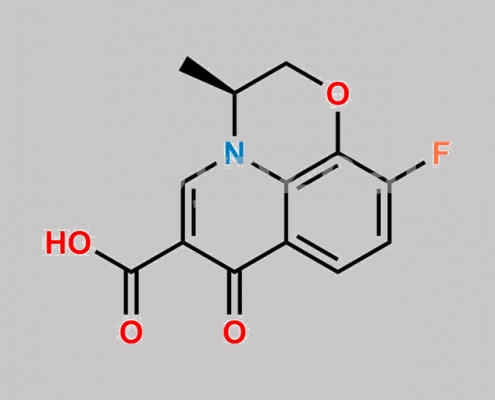
Levofloxacin 杂质 1 CAS号 117620-84-5
117620-84-5,Levofloxacin
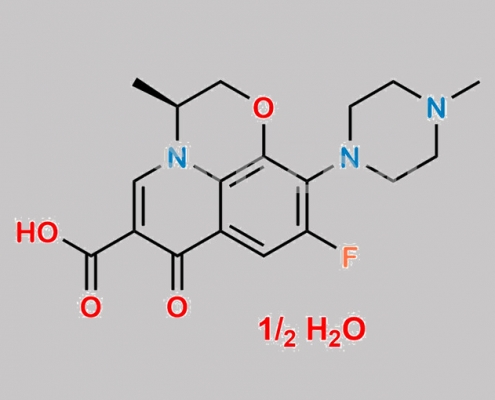
Levofloxacin Hemihydrate CAS号 138199-71-0
138199-71-0,Levofloxacin
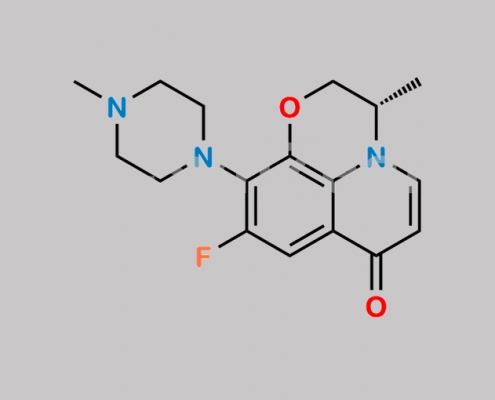
Levofloxacin EP Impurity E CAS号 178964-53-9
178964-53-9,Levofloxacin
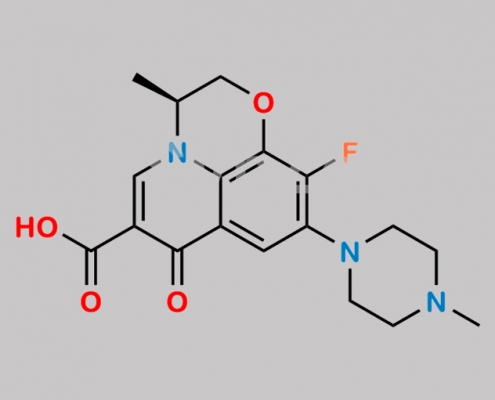
Levofloxacin EP Impurity I CAS号 178912-62-4
178912-62-4,Levofloxacin
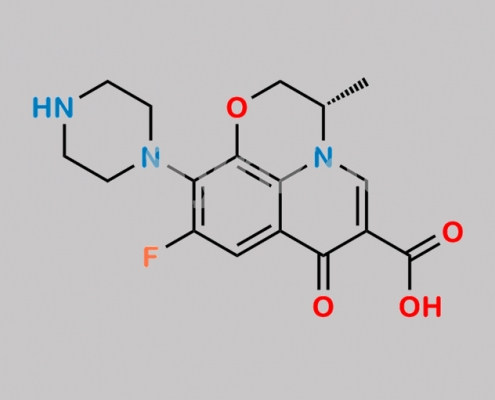
Levofloxacin EP Impurity B CAS号 117707-40-1
117707-40-1,Levofloxacin
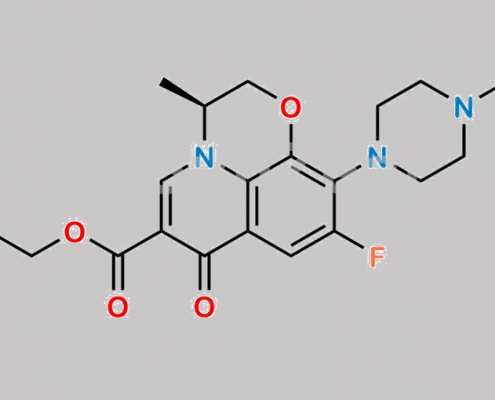
Levofloxacin EP Impurity H CAS号 177472-30-9
177472-30-9,Levofloxacin





