文章
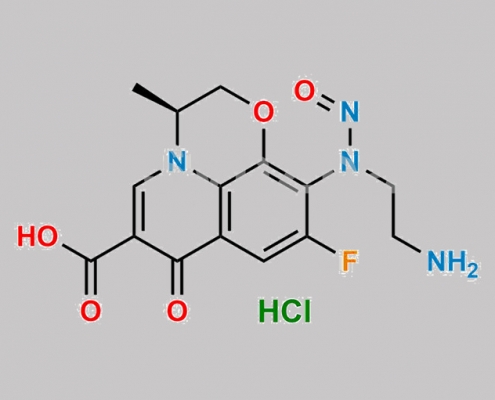
N-Nitroso-Levofloxacin 杂质-2 CAS号 N/A
N/A,Levofloxacin
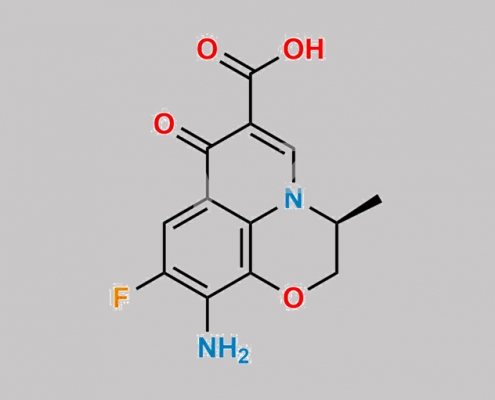
Levofloxacin 杂质 8 CAS号 151250-74-7
151250-74-7,Levofloxacin
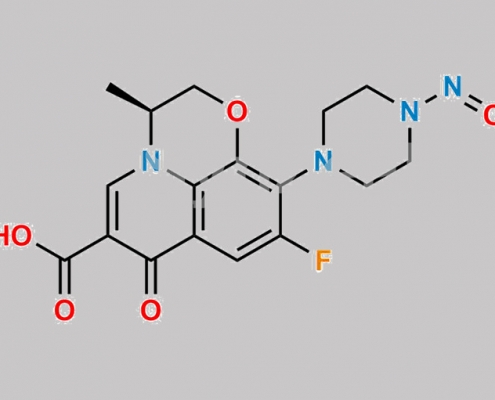
N-Nitroso Levofloxacin EP 杂质 B CAS号 1152314-62-9
1152314-62-9,Levofloxacin
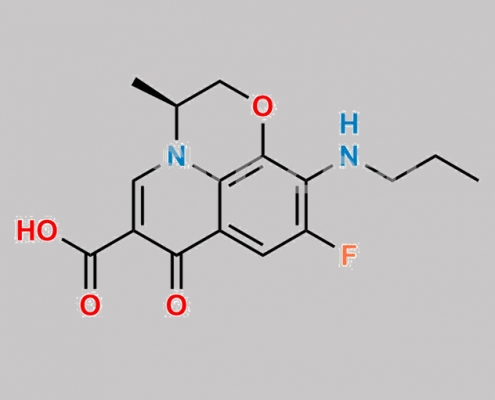
Levofloxacin 相关化合物 7 CAS号 1348712-95-7
1348712-95-7,Levofloxacin
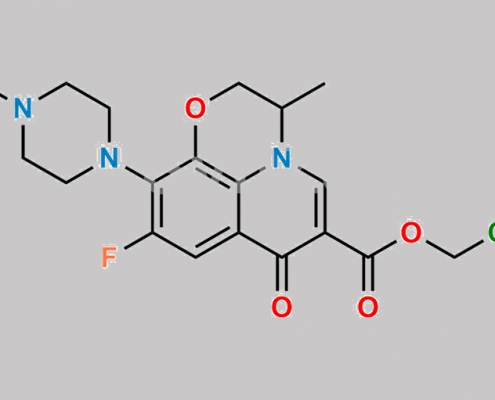
Levofloxacin 杂质 6 CAS号 N/A
N/A,Levofloxacin
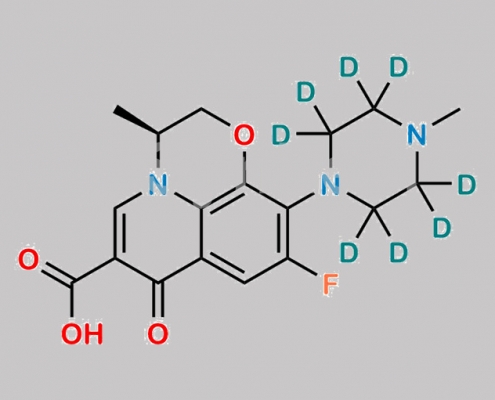
Levofloxacin D8 CAS号 1217716-71-6
1217716-71-6,Levofloxacin
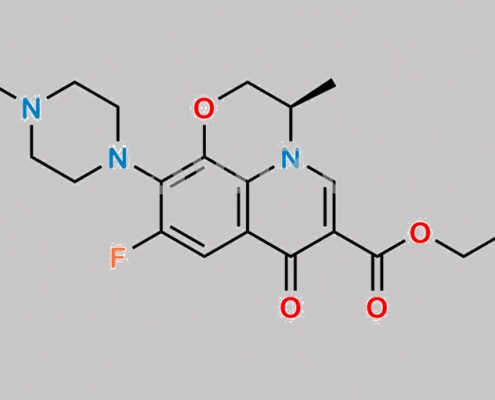
Levofloxacin 杂质 5 CAS号 2489671-30-7
2489671-30-7,Levofloxacin
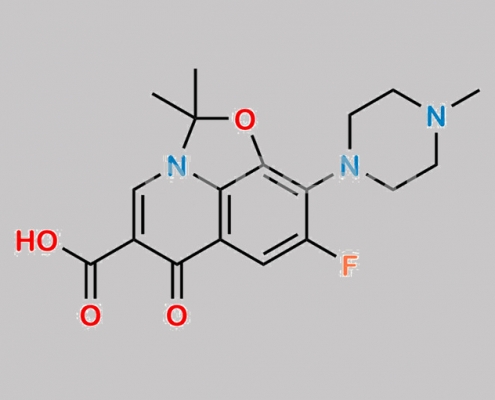
Levofloxacin 杂质 4 CAS号 2489671-28-3
2489671-28-3,Levofloxacin
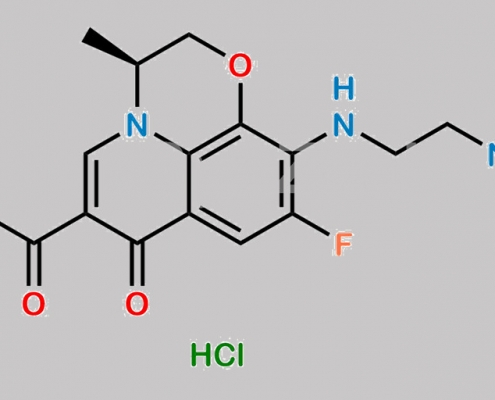
Levofloxacin Diamine 杂质 CAS号 1797099-76-3
1797099-76-3,Levofloxacin
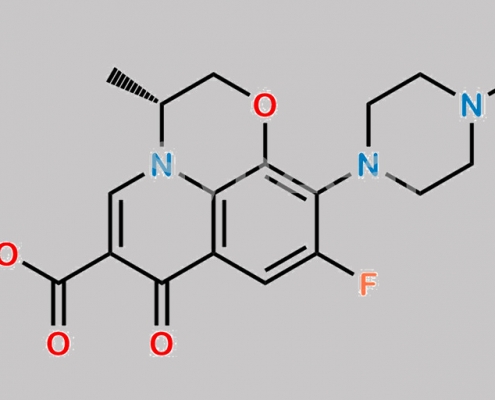
Levofloxacin EP 杂质 A CAS号 100986-86-5
100986-86-5,Levofloxacin





