文章
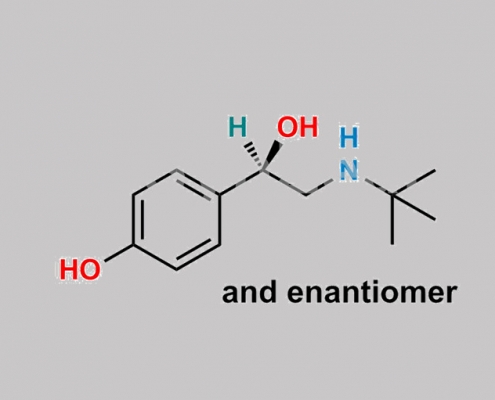
96948-64-0,Levalbuterol
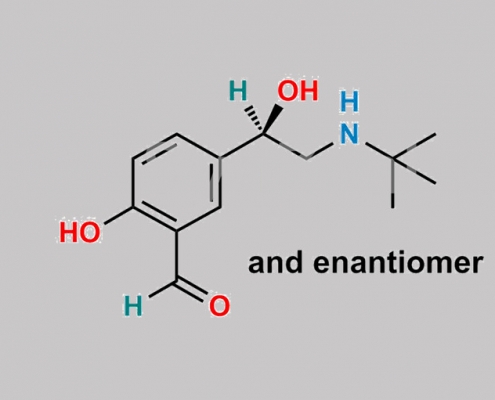
156339-88-7,Levalbuterol
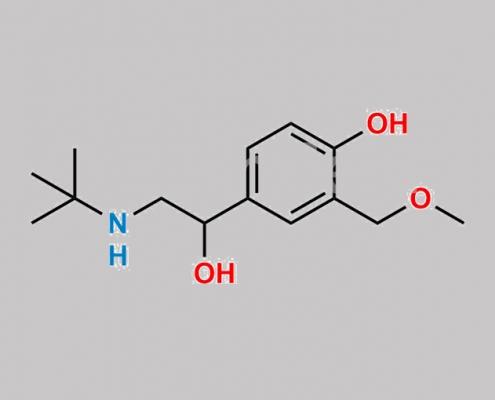
18910-70-8,Levalbuterol
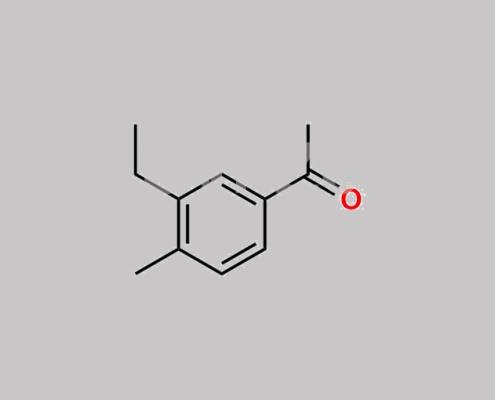
40180-56-1,Levalbuterol
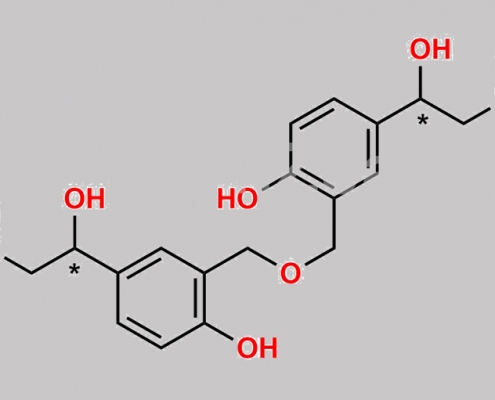
147663-30-7,Levalbuterol
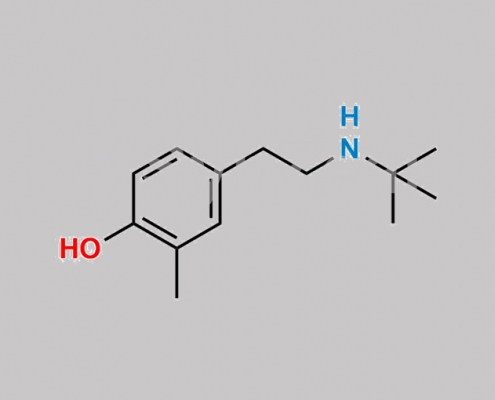
132183-64-3,Levalbuterol
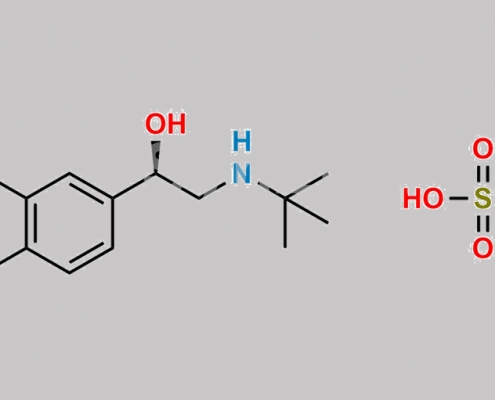
148563-16-0,Levalbuterol
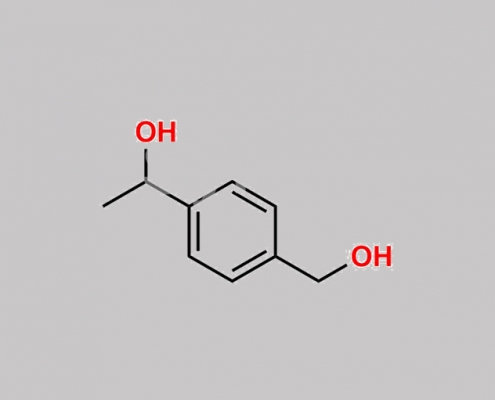
80463-22-5,Levalbuterol
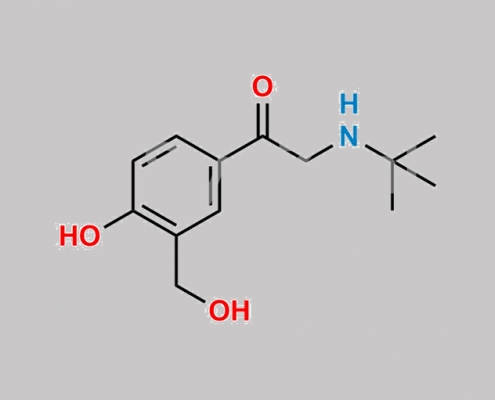
156547-62-5,Levalbuterol
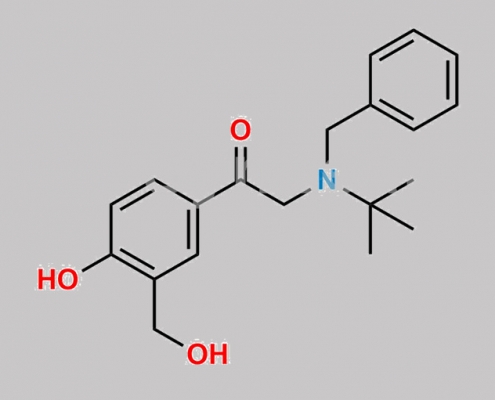
64092-10-0,Levalbuterol
Scroll to top















