文章
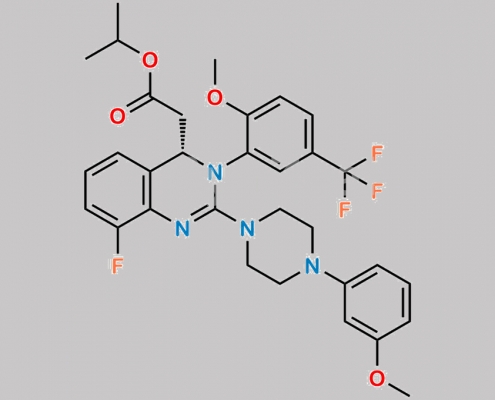
Letermovir 杂质 5 CAS号 2872586-94-0
2872586-94-0,Letermovir
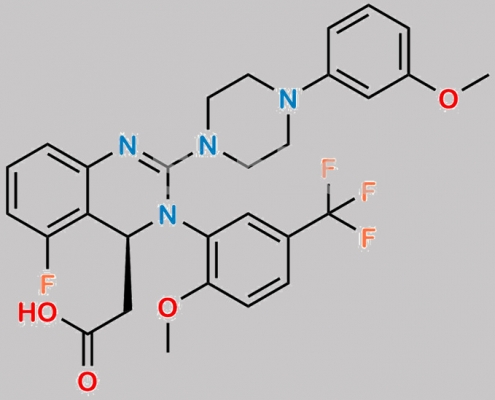
3-Fluoro Letermovir CAS号 N/A
N/A,Letermovir
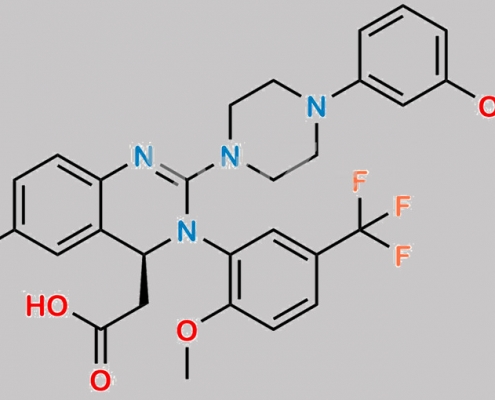
4-Fluoro Letermovir CAS号 N/A
N/A,Letermovir
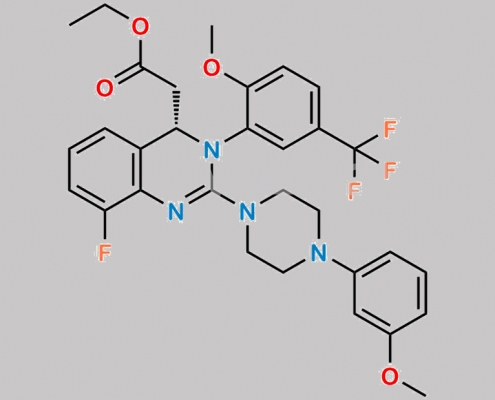
Letermovir Ethyl Ester CAS号 2758792-79-7
2758792-79-7,Letermovir
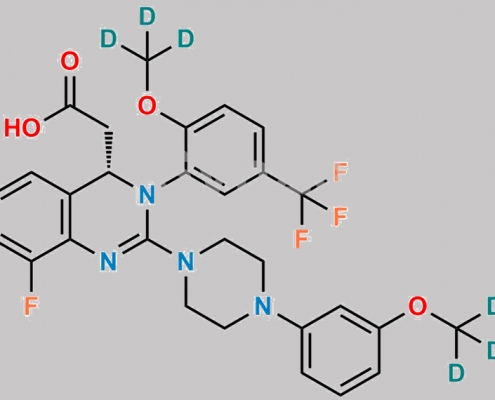
Letermovir d6 CAS号 N/A
N/A,Letermovir
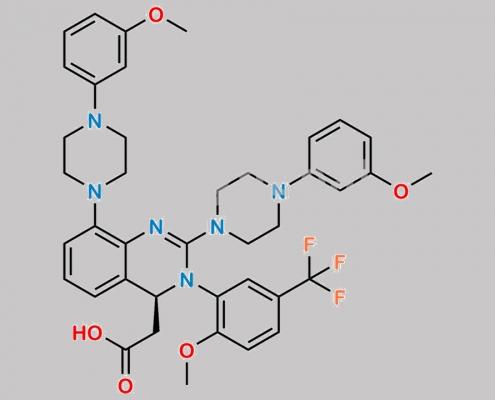
Letermovir 杂质 1 CAS号 N/A
N/A,Letermovir
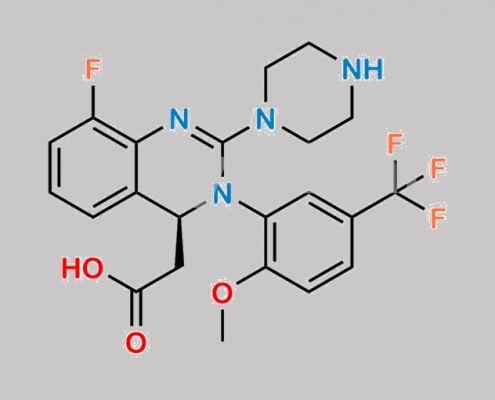
Letermovir 杂质 4 CAS号 N/A
N/A,Letermovir
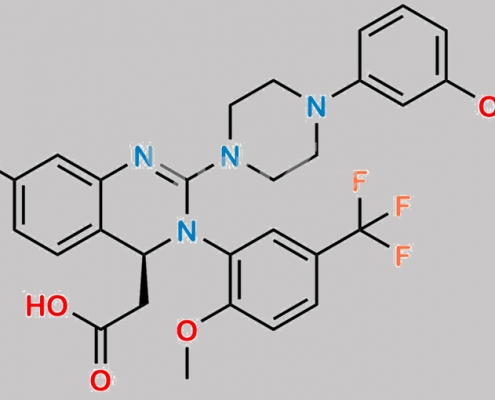
5-Fluoro Letermovir CAS号 N/A
N/A,Letermovir
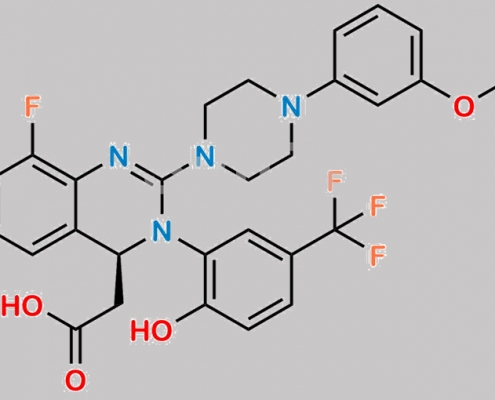
Letermovir 杂质 2 CAS号 N/A
N/A,Letermovir
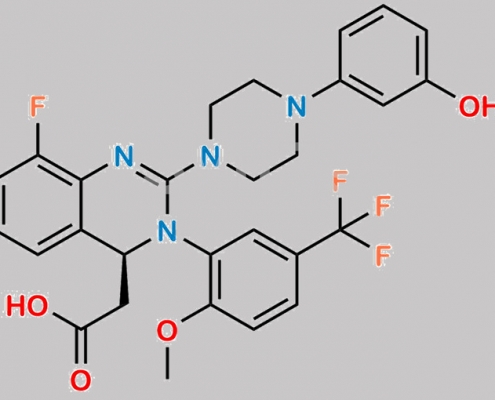
Letermovir 杂质 3 CAS号 N/A
N/A,Letermovir





