文章
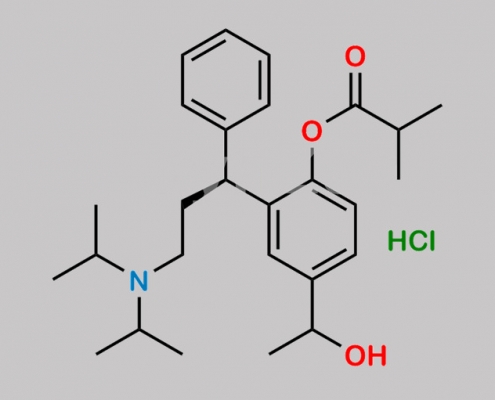
Fesoterodine Related Impurity 7 HCl CAS号 N/A
N/A,Fesoterodine
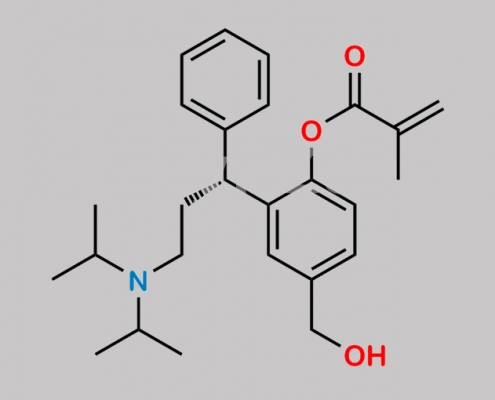
Fesoterodine Related Impurity 10 CAS号 1390644-37-7
1390644-37-7,Fesoterodine
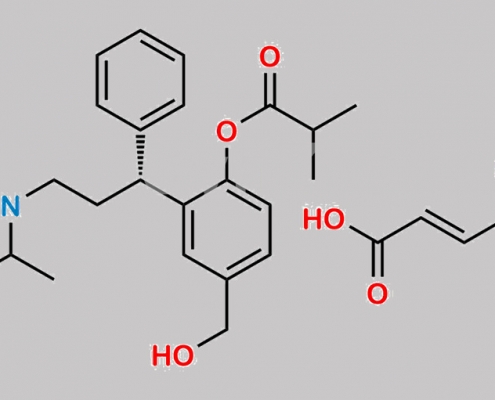
Fesoterodine Fumarate CAS号 286930-03-8
286930-03-8,Fesoterodine
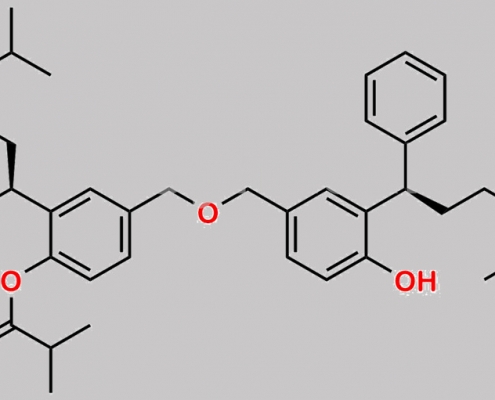
Monoester of Symmetrical Dimer CAS号 N/A
N/A,Fesoterodine
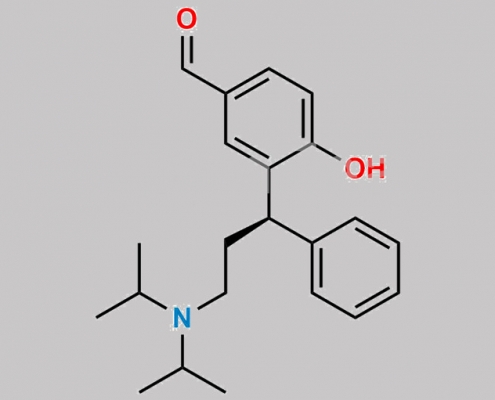
Fesoterodine Aldehyde of Diol Fumarate CAS号 N/A
N/A,Fesoterodine
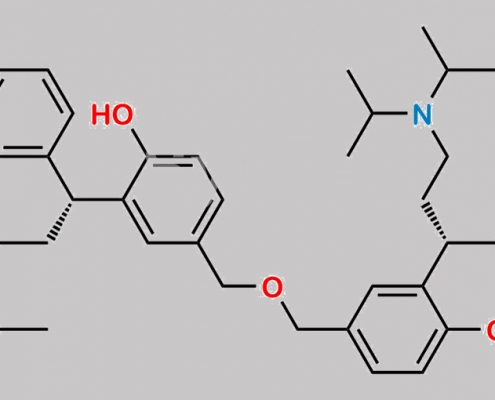
Symmetrical Dimer of Diol CAS号 N/A
N/A,Fesoterodine
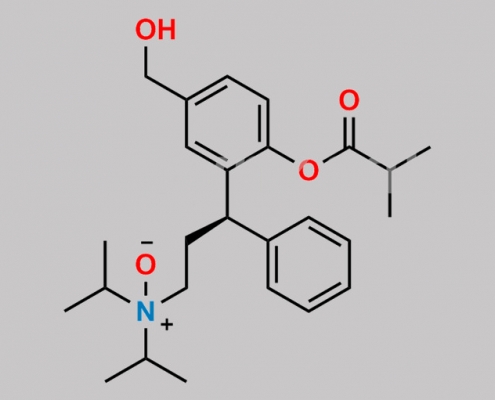
Fesoterodine N-Oxide CAS号 N/A
N/A,Fesoterodine
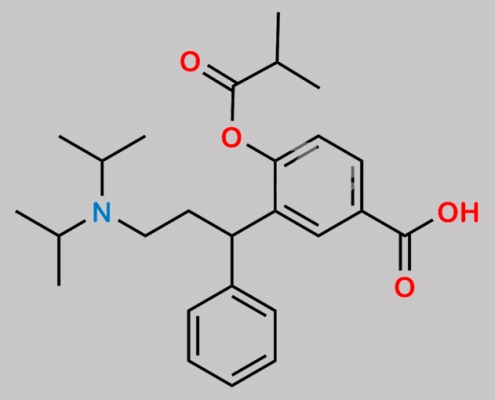
Fesoterodine Impurity D CAS号 1262778-55-1
1262778-55-1,Fesoterodine
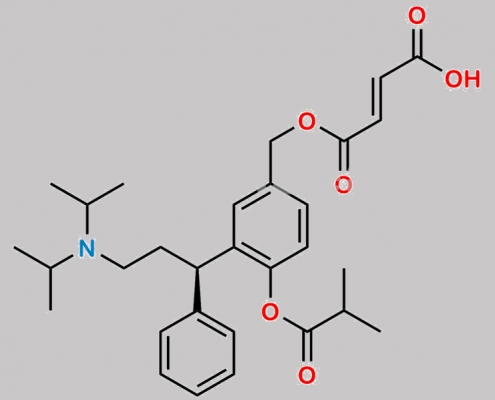
Fesoterodine Impurity G CAS号 1254942-29-4
1254942-29-4,Fesoterodine

Fesoterodine Related Impurity 4 CAS号 2765390-15-4
2765390-15-4,Fesoterodine





