标签存档: Favipiravir
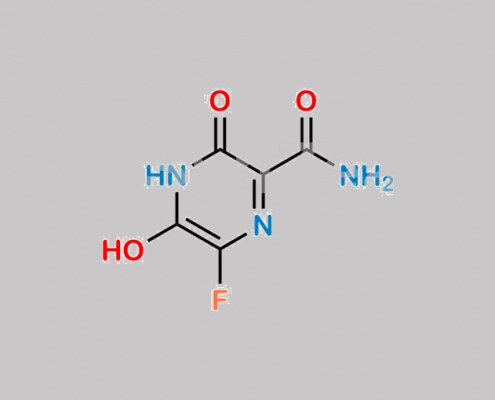
1492021-29-0,Favipiravir
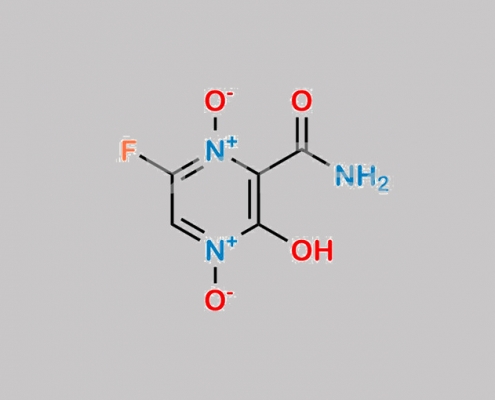
2809442-05-3,Favipiravir
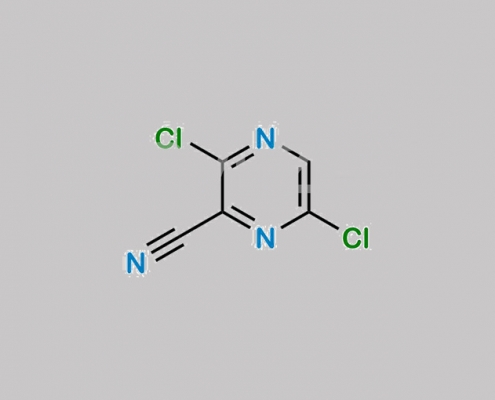
356783-16-9,Favipiravir
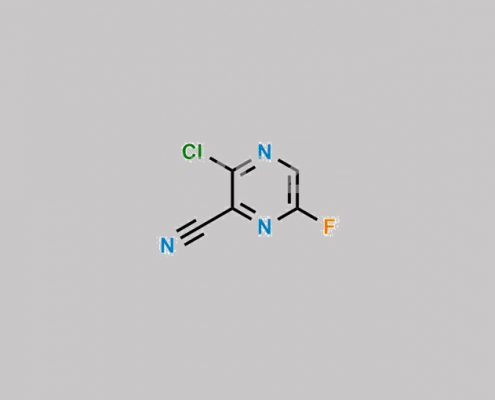
356783-49-8,Favipiravir
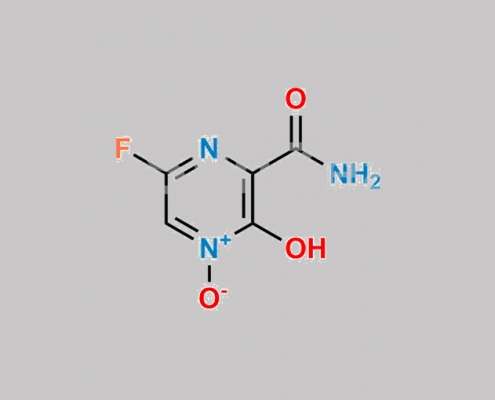
2809392-10-5,Favipiravir
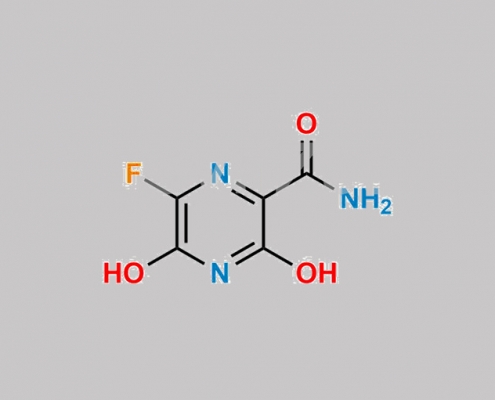 https://www.watson-int.cn/wp-content/uploads/2024/07/favipiravir-impurity-23.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 10:59:062024-07-08 10:59:06Favipiravir 杂质 23 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/favipiravir-impurity-23.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 10:59:062024-07-08 10:59:06Favipiravir 杂质 23 CAS号 N/A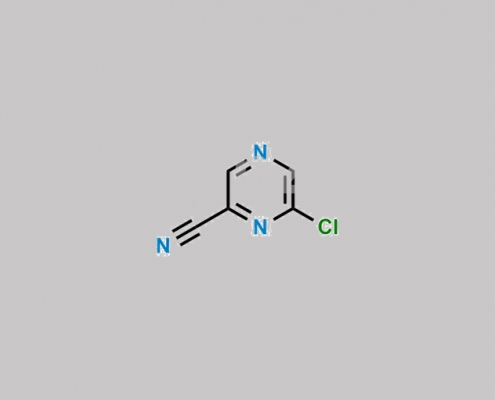
6863-74-7,Favipiravir
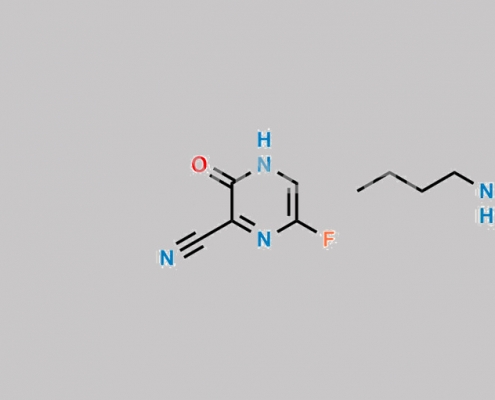
1137606-76-8,Favipiravir
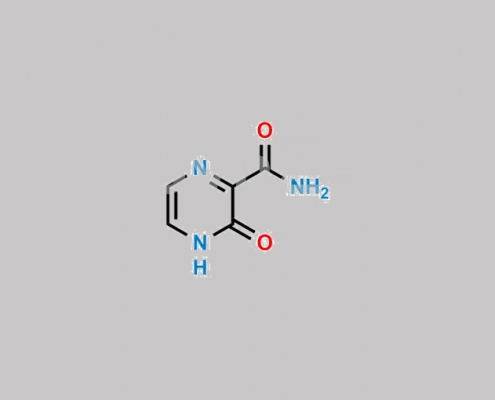
55321-99-8,Favipiravir
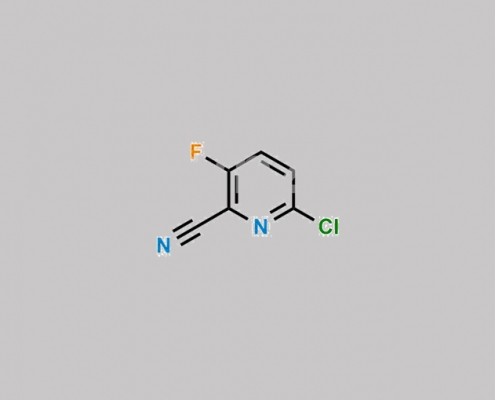
1207609-52-6,Favipiravir
Scroll to top















