文章
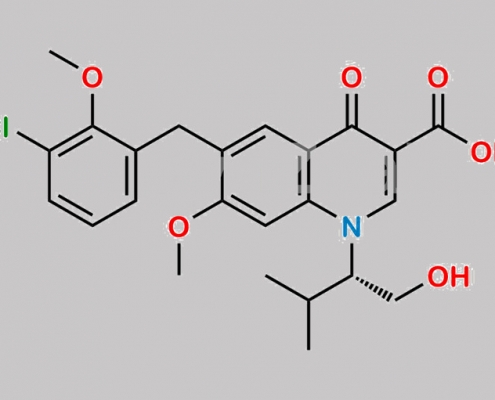
N/A,Elvitegravir
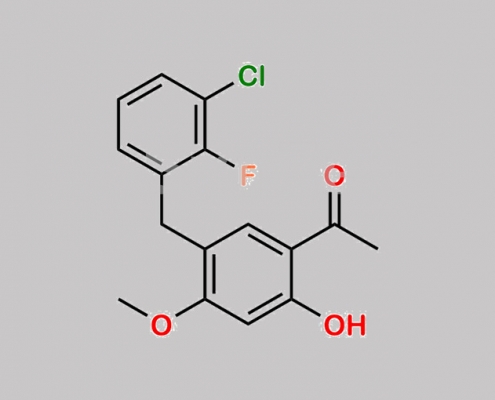
N/A,Elvitegravir
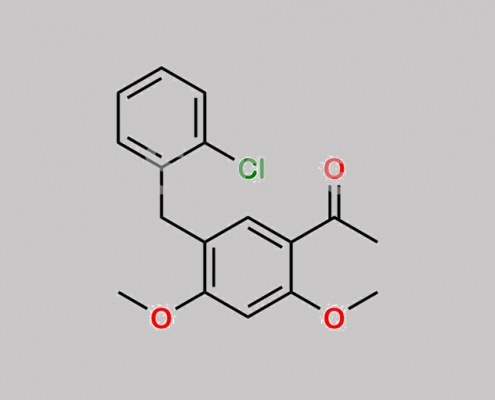
N/A,Elvitegravir
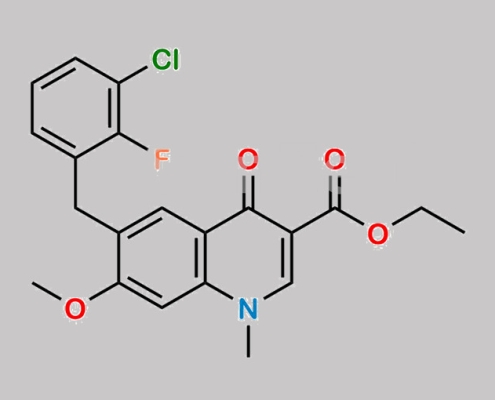
N/A,Elvitegravir
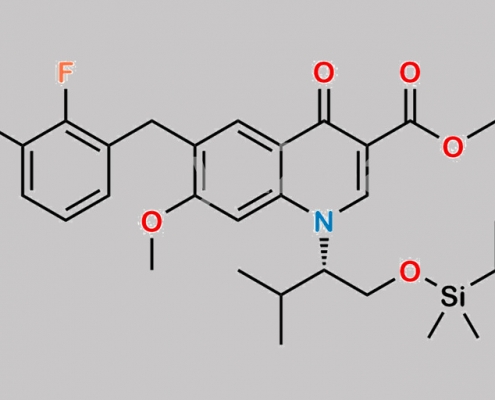
949465-85-4,Elvitegravir
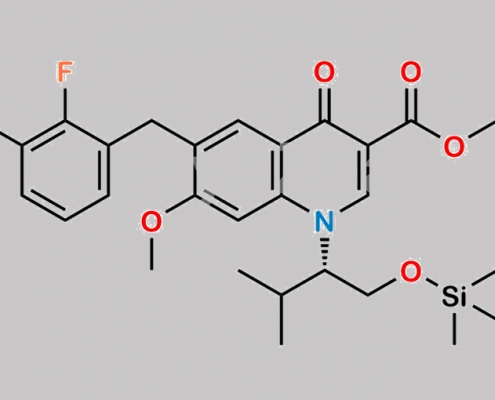
N/A,Elvitegravir
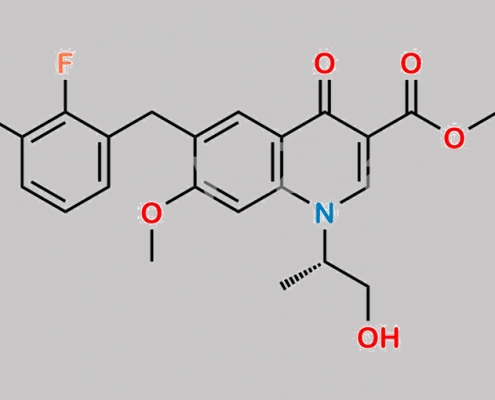
N/A,Elvitegravir
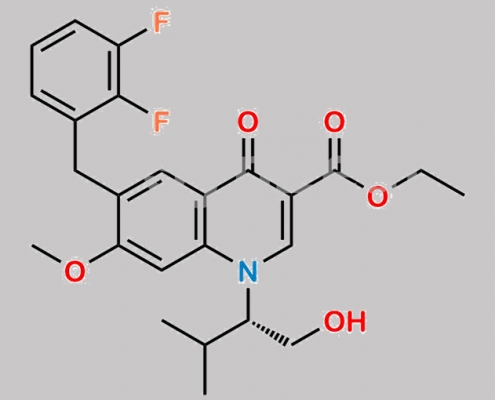
N/A,Elvitegravir
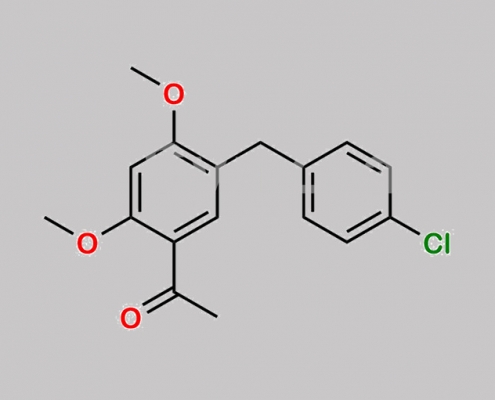
N/A,Elvitegravir
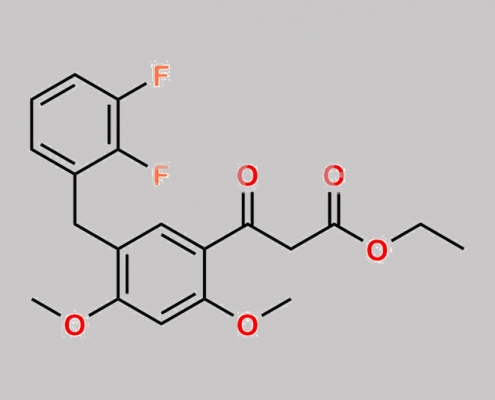
N/A,Elvitegravir
Scroll to top















