文章
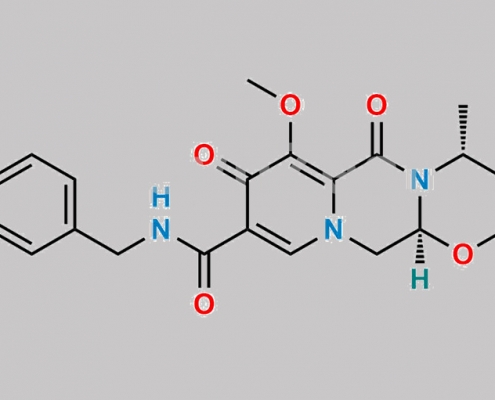
Dolutegravir 杂质 24 CAS号 2244245-34-7
2244245-34-7,Dolutegravir
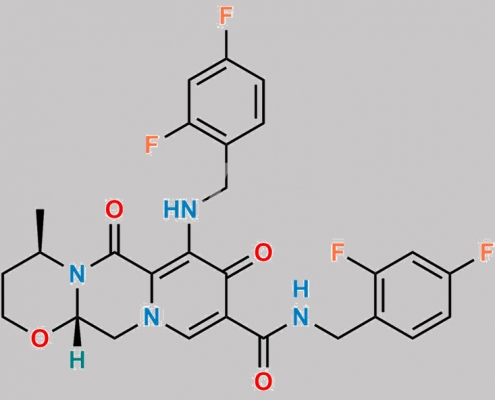
Dolutegravir 杂质 26 CAS号 N/A
N/A,Dolutegravir
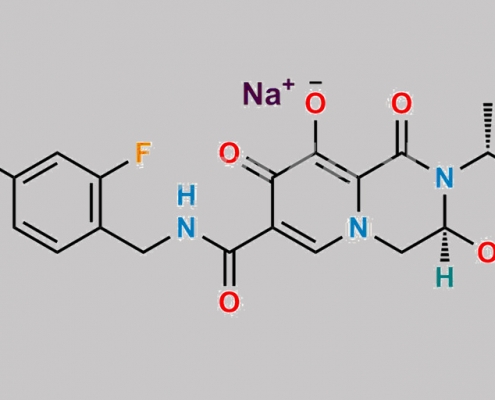
Dolutegravir Sodium CAS号 1051375-19-9
1051375-19-9,Dolutegravir
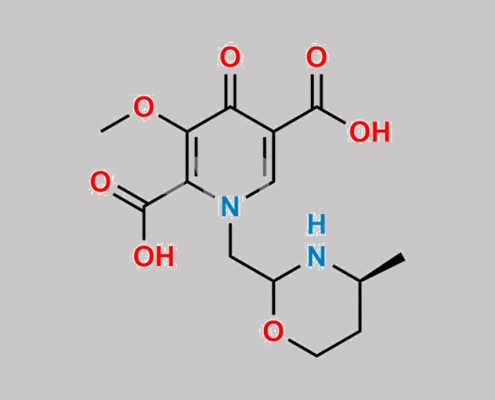
Dolutegravir 杂质 20 CAS号 N/A
N/A,Dolutegravir
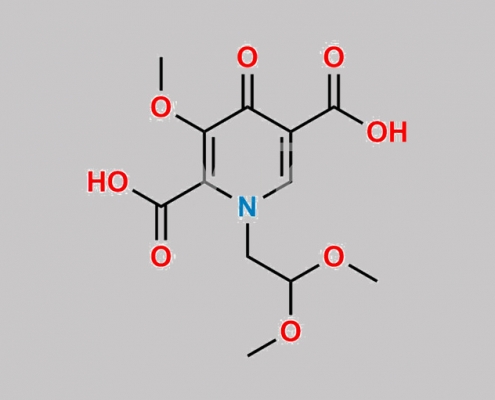
Dolutegravir 杂质 16 CAS号 2651309-06-5
2651309-06-5,Dolutegravir
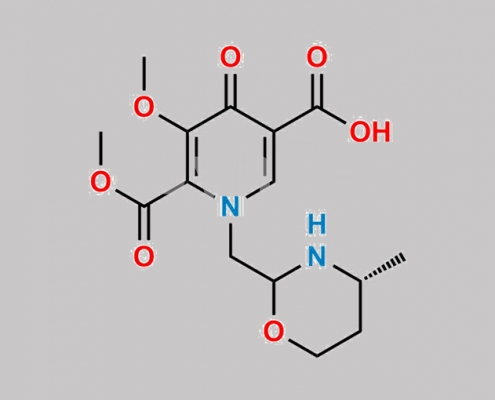
Dolutegravir 杂质 17 CAS号 N/A
N/A,Dolutegravir
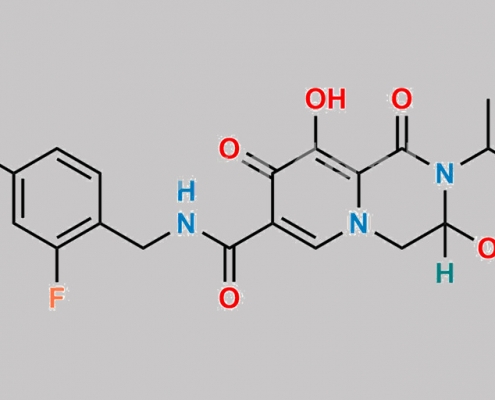
Racemic Dolutegravir CAS号 N/A
N/A,Dolutegravir
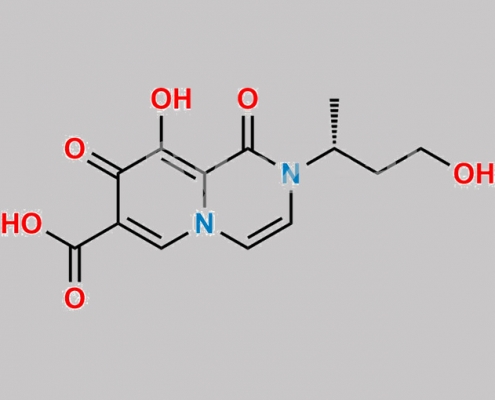
Dolutegravir 杂质 19 CAS号 N/A
N/A,Dolutegravir
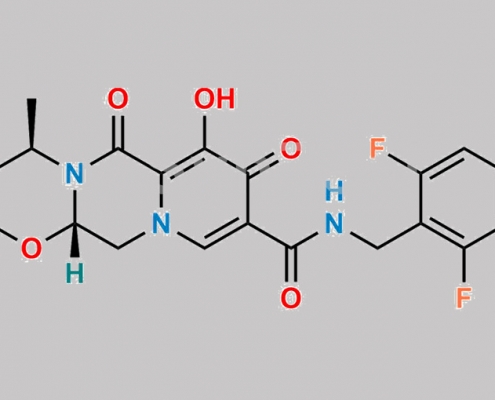
4-Desfluoro-6-fluoro Dolutegravir CAS号 2244161-72-4
2244161-72-4,Dolutegravir
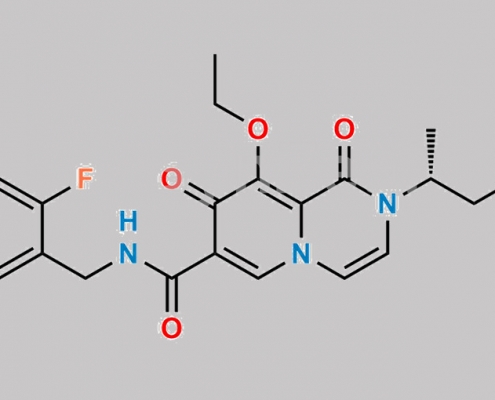
Dolutegravir 杂质 14 CAS号 2778347-47-8
2778347-47-8,Dolutegravir





