文章
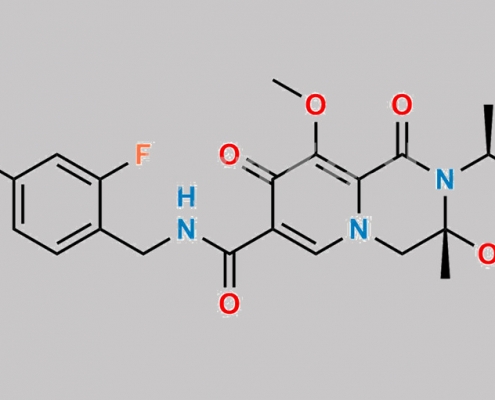
Dolutegravir 杂质 32 CAS号 N/A
N/A,Dolutegravir
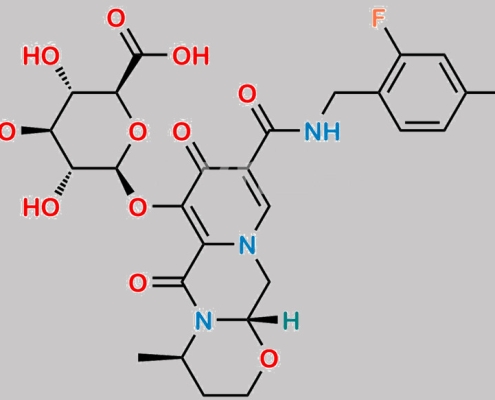
Dolutegravir O-beta-D-Glucuronide CAS号 1485692-21-4
1485692-21-4,Dolutegravir
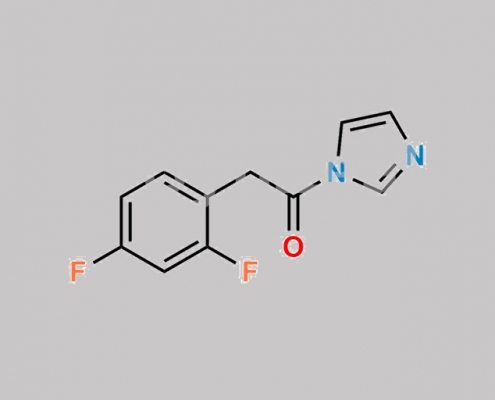
Dolutegravir 杂质 28 CAS号 603999-55-9
603999-55-9,Dolutegravir
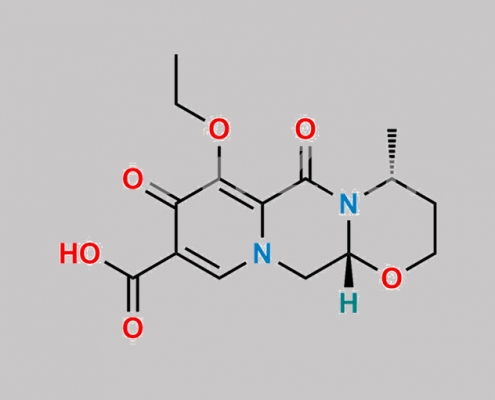
Dolutegravir 杂质 31 CAS号 N/A
N/A,Dolutegravir
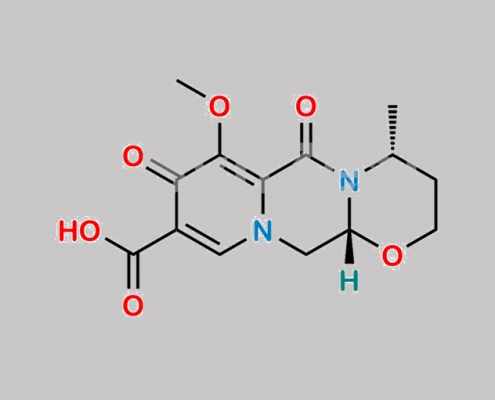
Dolutegravir 杂质 29 CAS号 2315439-81-5
2315439-81-5,Dolutegravir
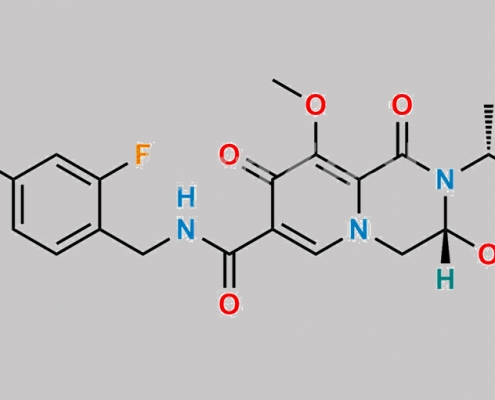
Dolutegravir 杂质 23 CAS号 2384108-28-3
2384108-28-3,Dolutegravir
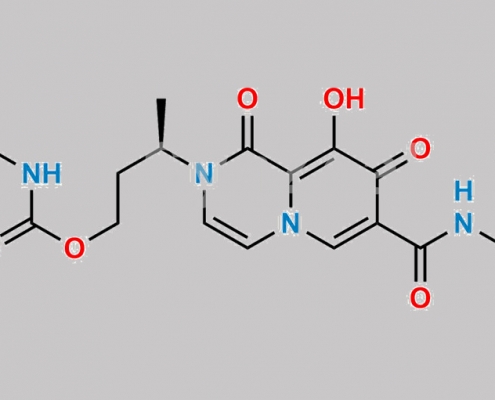
Dolutegravir 杂质 25 CAS号 2244161-73-5
2244161-73-5,Dolutegravir
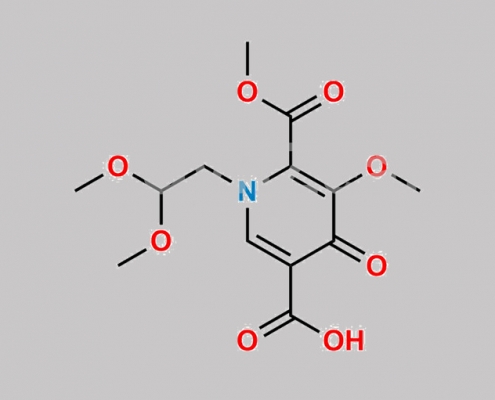
Dolutegravir 杂质 22 CAS号 1335210-23-5
1335210-23-5,Dolutegravir
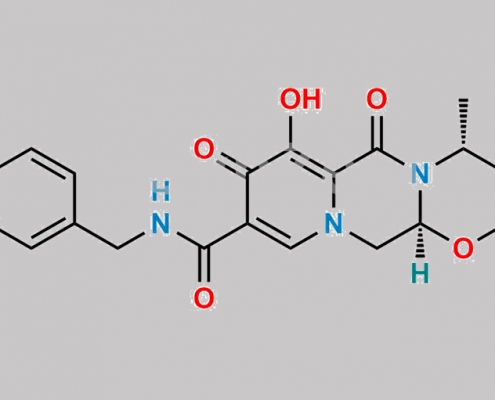
Desdifluoro Dolutegravir CAS号 2244161-71-3
2244161-71-3,Dolutegravir
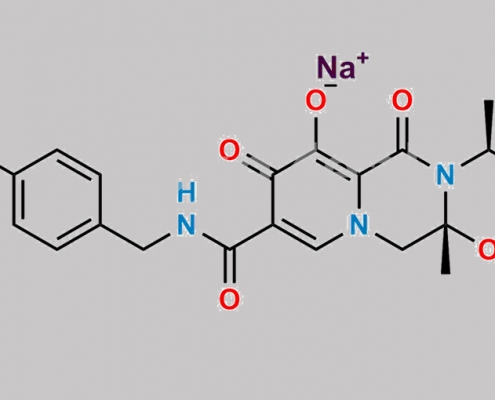
Dolutegravir 杂质 27 CAS号 N/A
N/A,Dolutegravir





