文章
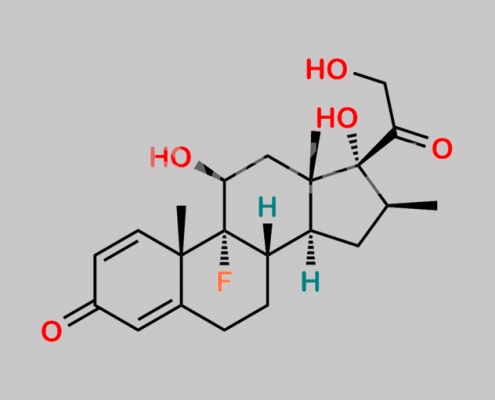
Dexamethasone EP 杂质 B CAS号 378-44-9
378-44-9,Dexamethasone
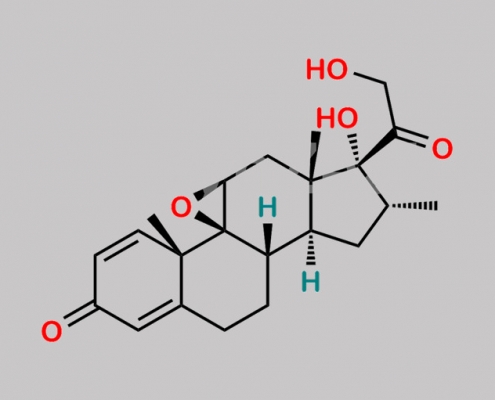
Dexamethasone EP 杂质 D CAS号 24916-90-3
24916-90-3,Dexamethasone
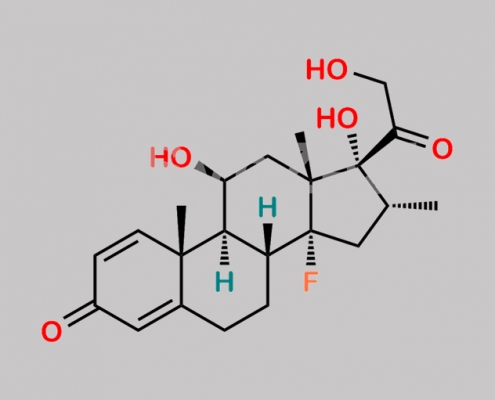
Dexamethasone EP 杂质 A CAS号 N/A
N/A,Dexamethasone
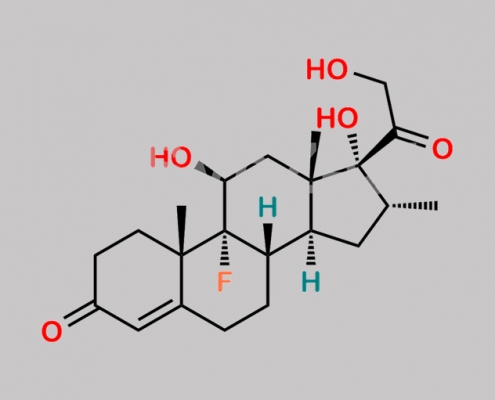
Dexamethasone EP 杂质 C CAS号 426-17-5
426-17-5,Dexamethasone
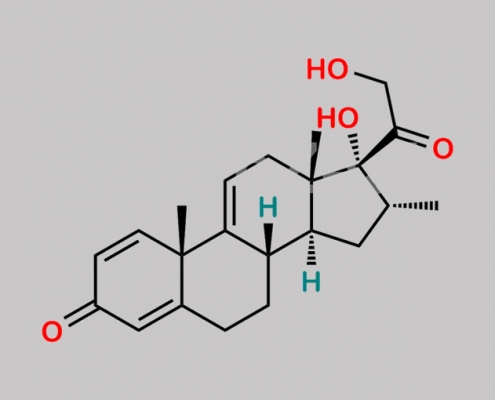
Dexamethasone EP 杂质 E CAS号 13209-41-1
13209-41-1,Dexamethasone
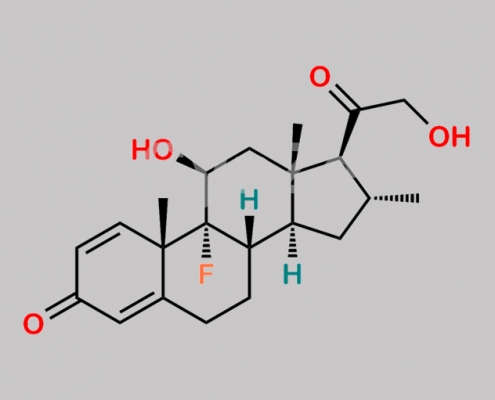
Dexamethasone EP 杂质 F CAS号 382-67-2
382-67-2,Dexamethasone
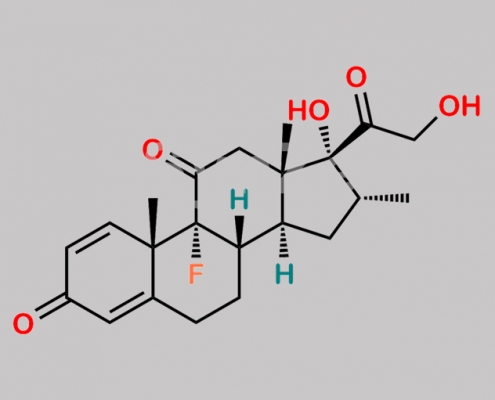
11-Dehydrodexamethasone CAS号 2964-81-0
2964-81-0,Dexamethasone
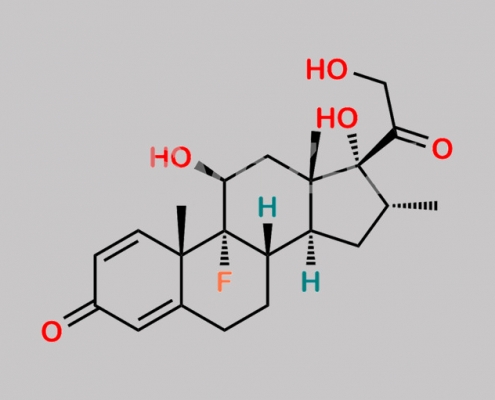
Dexamethasone CAS号 50-02-2
50-02-2,Dexamethasone
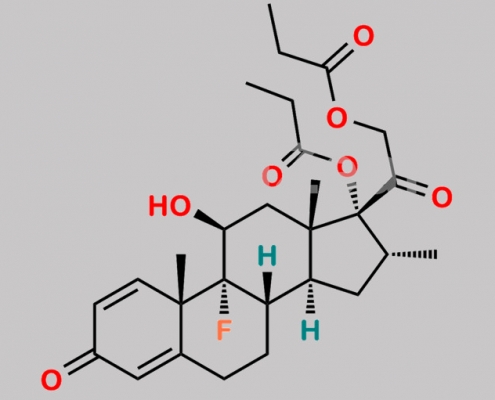
Dexamethasone-17,21-dipropionate CAS号 55541-30-5
55541-30-5,Dexamethasone
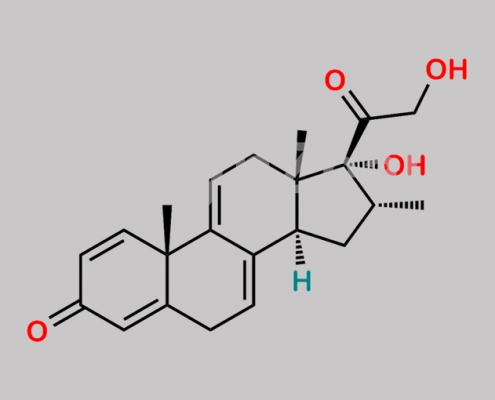
Dexamethasone EP 杂质 K CAS号 1809224-82-5
1809224-82-5,Dexamethasone





