文章
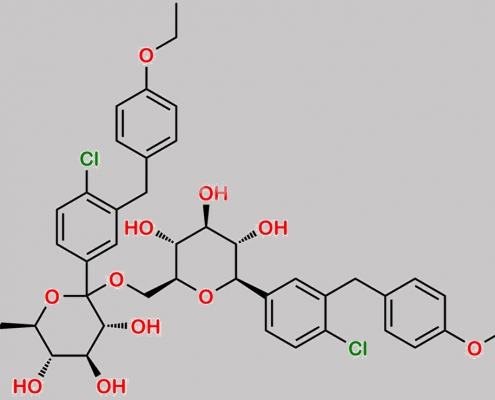
Dapagliflozin 杂质 24 CAS号 N/A
N/A,Dapagliflozin
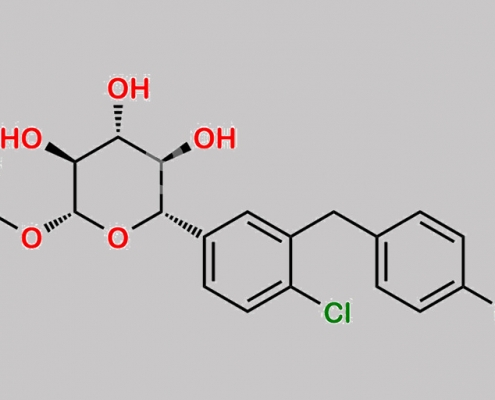
Dapagliflozin 杂质 73 CAS号 N/A
N/A,Dapagliflozin

Dapagliflozin 杂质 59 CAS号 N/A
N/A,Dapagliflozin
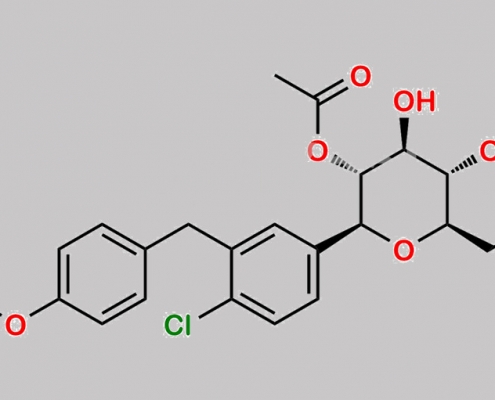
Dapagliflozin 杂质 68 CAS号 N/A
N/A,Dapagliflozin
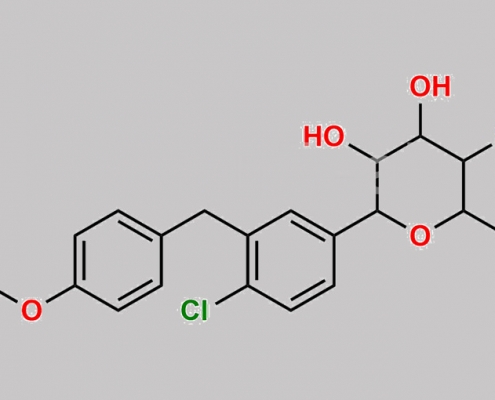
Dapagliflozin 杂质 50 CAS号 N/A
N/A,Dapagliflozin
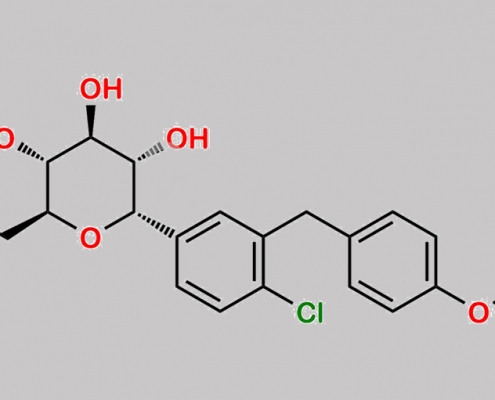
Dapagliflozin 杂质 61 CAS号 2452300-81-9
2452300-81-9,Dapagliflozin

Dapagliflozin 杂质 56 CAS号 21900-52-7
21900-52-7,Dapagliflozin

Dapagliflozin 杂质 64 CAS号 N/A
N/A,Dapagliflozin
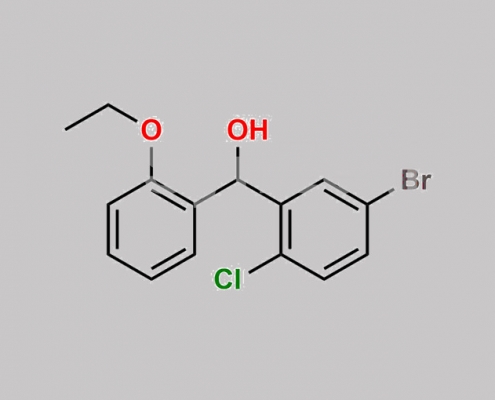
Dapagliflozin 杂质 20 CAS号 1713991-97-9
1713991-97-9,Dapagliflozin
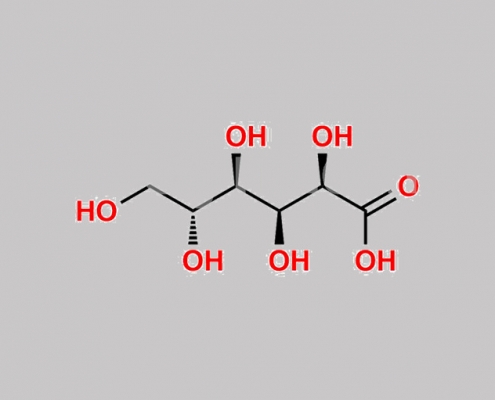
Dapagliflozin 杂质 38 CAS号 526-95-4
526-95-4,Dapagliflozin





