文章
 https://www.watson-int.cn/wp-content/uploads/2024/07/daclatasvir-nitroso-impurity-2.jpg
606
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
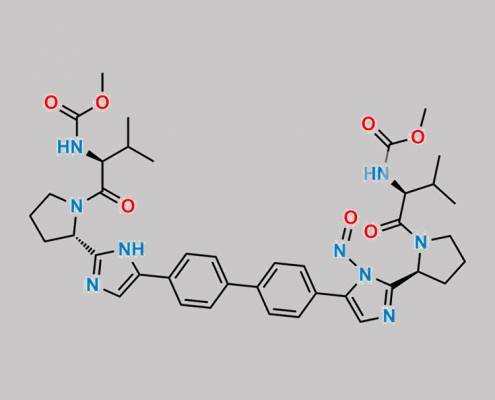
great_watson-int2024-07-08 07:35:362024-07-08 07:35:36Daclatasvir Nitroso 杂质 2 CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/daclatasvir-nitroso-impurity-2.jpg
606
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 07:35:362024-07-08 07:35:36Daclatasvir Nitroso 杂质 2 CAS号 N/A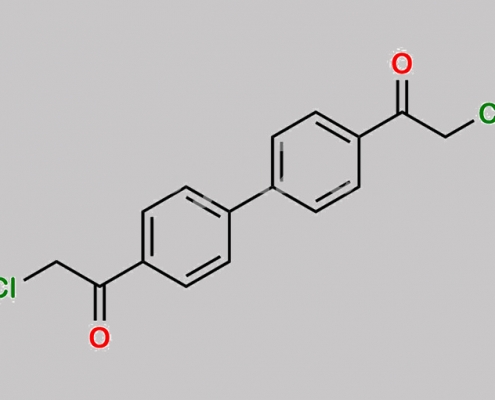
24860-53-5,Daclatasvir

15761-39-4,Daclatasvir
1009119-65-6,Daclatasvir

4072-67-7,Daclatasvir
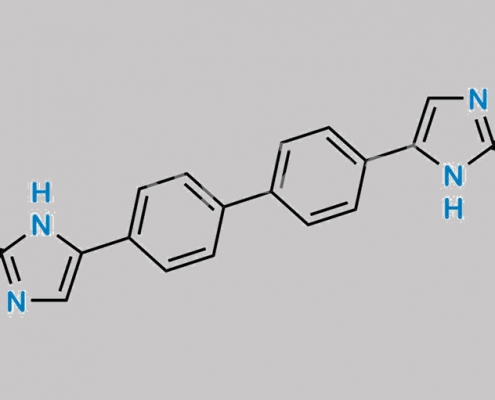
1007882-23-6,Daclatasvir
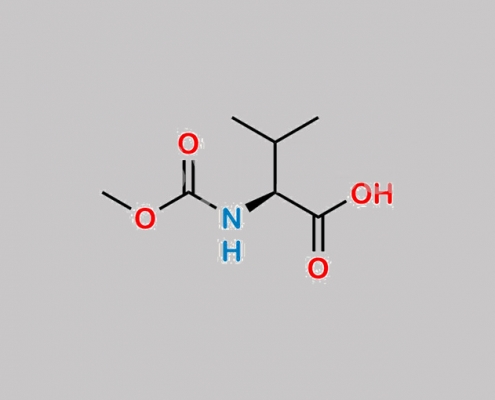
74761-42-5,Daclatasvir
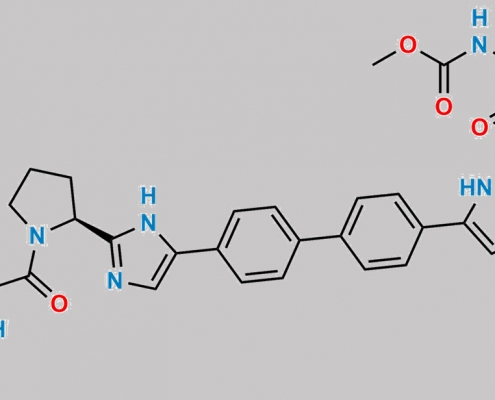
2077207-88-4,Daclatasvir
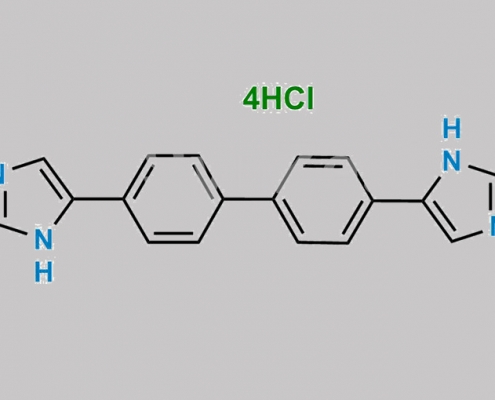 https://www.watson-int.cn/wp-content/uploads/2024/07/daclatasvir-impurity-8.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
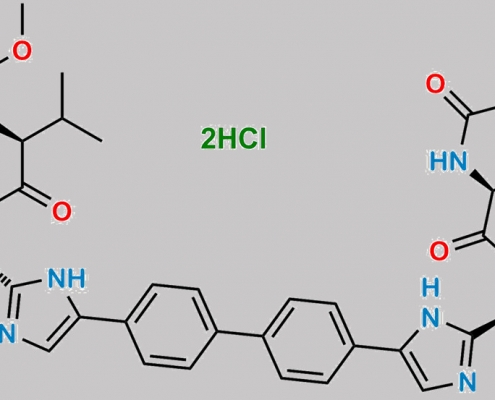
great_watson-int2024-07-08 05:50:492024-07-08 05:50:49Daclatasvir 杂质 8 (4HCl) CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/daclatasvir-impurity-8.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-08 05:50:492024-07-08 05:50:49Daclatasvir 杂质 8 (4HCl) CAS号 N/A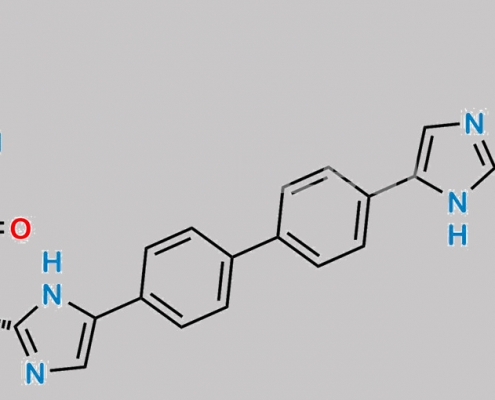
1007884-60-7,Daclatasvir
Scroll to top















