文章
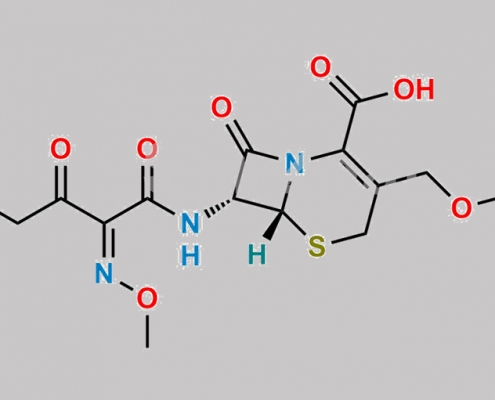
Cefotaxime Bromoacetyl Analog CAS号 83305-12-8
83305-12-8,Cefotaxime Sodium
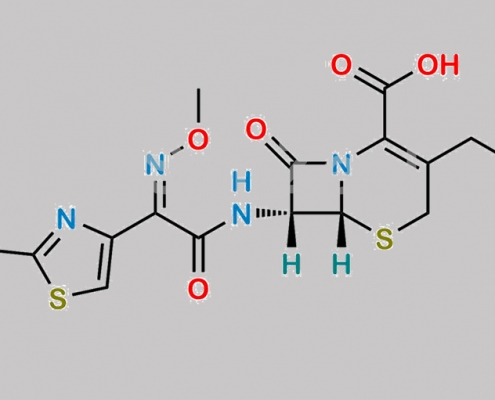
Cefotaxime Sodium EP 杂质 C CAS号 66403-32-5
66403-32-5,Cefotaxime Sodium
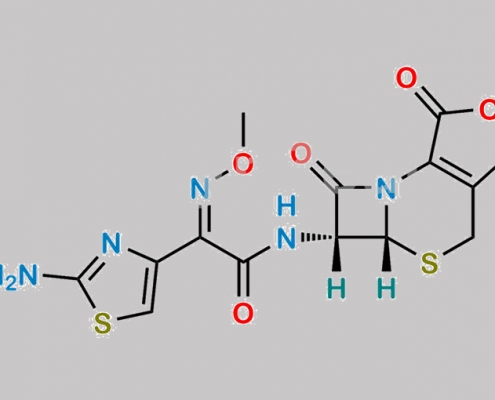
Cefotaxime Sodium EP 杂质 E CAS号 66340-33-8
66340-33-8,Cefotaxime Sodium
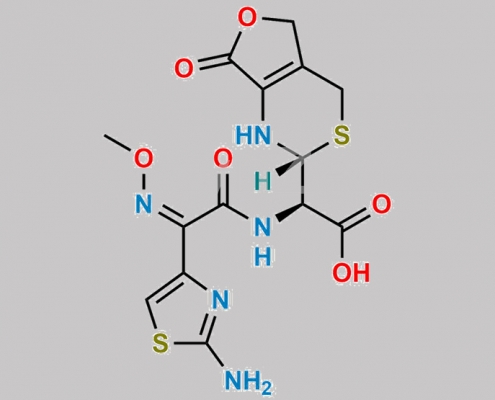
Cefotaxime Open Ring Lactone CAS号 75679-12-8
75679-12-8,Cefotaxime Sodium
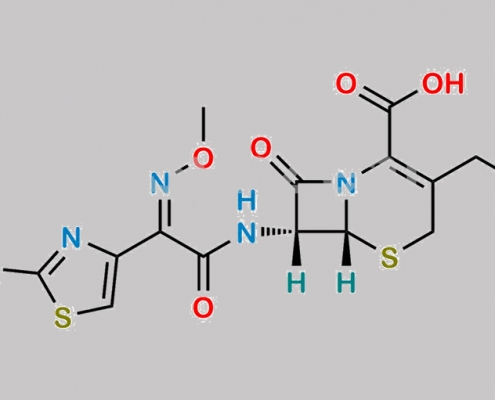
Cefotaxime Sodium EP 杂质 B CAS号 66340-28-1
66340-28-1,Cefotaxime Sodium
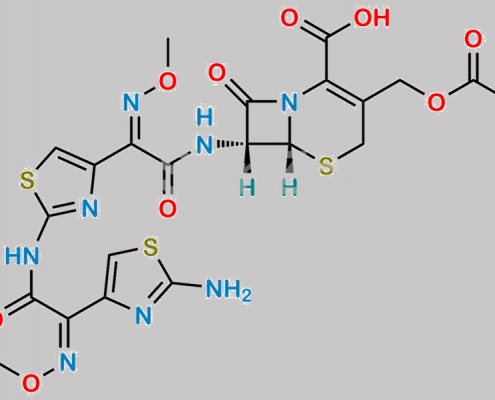
Cefotaxime Sodium EP 杂质 G CAS号 N/A
N/A,Cefotaxime Sodium
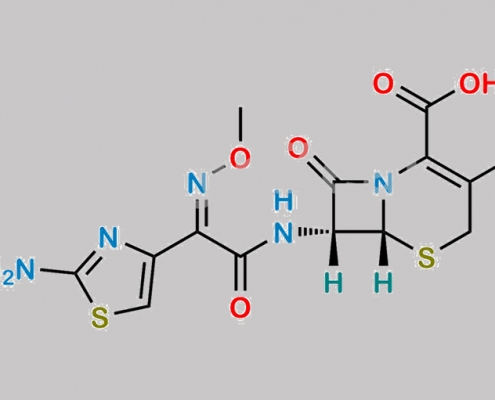
Cefotaxime Sodium EP 杂质 A CAS号 65052-63-3
65052-63-3,Cefotaxime Sodium
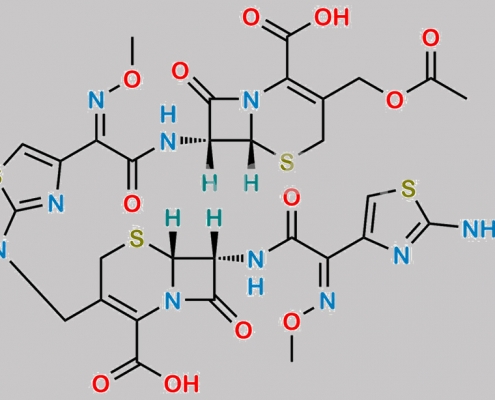
Cefotaxime Sodium EP 杂质 F CAS号 175032-97-0
175032-97-0,Cefotaxime Sodium
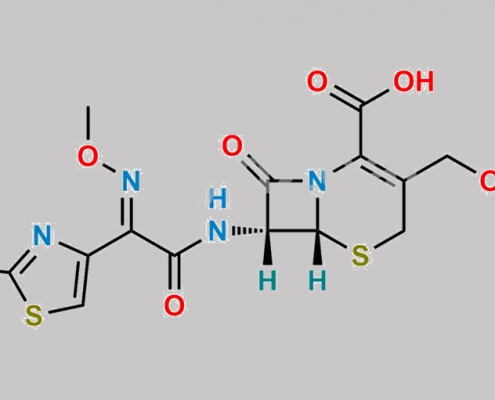
Cefotaxime Sodium EP 杂质 D CAS号 63527-53-7
63527-53-7,Cefotaxime Sodium
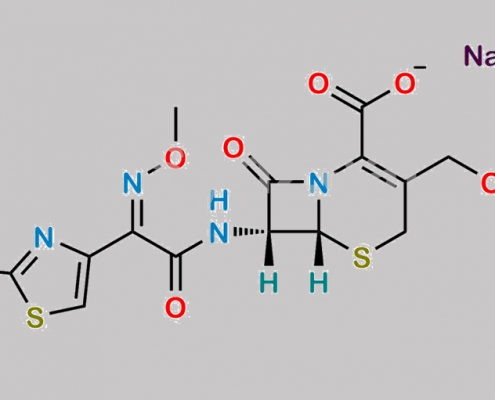
Cefotaxime Sodium CAS号 64485-93-4
64485-93-4,Cefotaxime Sodium





