文章 2024年7月8日
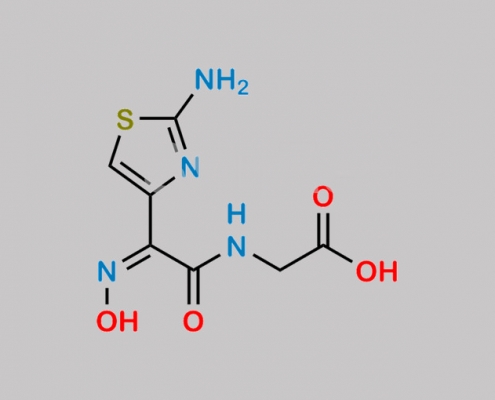
178601-89-3,Cefdinir
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-7-epimer.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
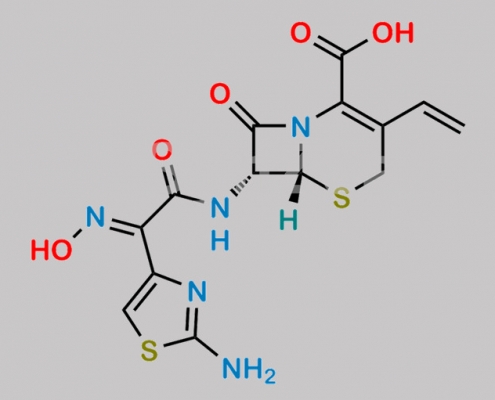
great_watson-int 2024-07-08 00:05:28 2024-07-08 00:05:28 Cefdinir 7-Epimer CAS号 178601-89-3 2024年7月7日
178949-04-7,Cefdinir
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-decarboxy-open-ring-lactone-impurity.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-07 00:15:08 2024-07-07 00:15:08 Cefdinir Decarboxy Open Ring Lactone Impurity CAS号 178949-04-7 2024年7月6日
946573-41-7,Cefdinir
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-lactone-usp.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
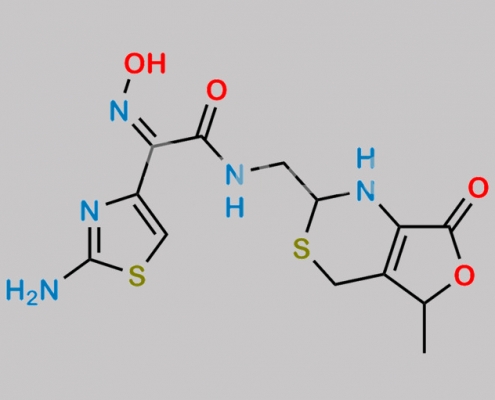
great_watson-int 2024-07-06 23:03:43 2024-07-06 23:03:43 Cefdinir Lactone (USP) CAS号 946573-41-7 2024年7月6日
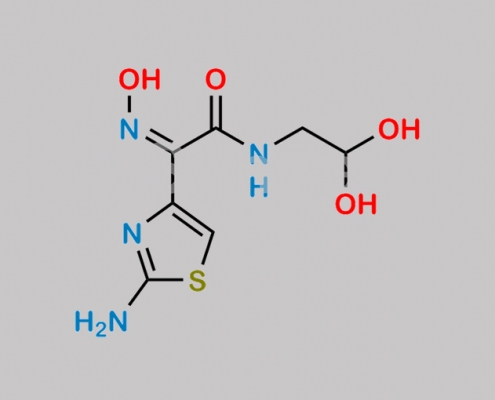
178422-40-7,Cefdinir
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-thiazolylacetyl-glycine-oxime-acetal-impurity-usp.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-06 22:58:55 2024-07-06 22:58:55 Cefdinir Thiazolylacetyl Glycine Oxime Acetal Impurity (USP) CAS号 178422-40-7
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-usp-related-compound-asodium-salt.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-06 22:58:55 2024-07-06 22:58:55 Cefdinir USP Related Compound A (Sodium salt) CAS号 N/A 2024年7月6日
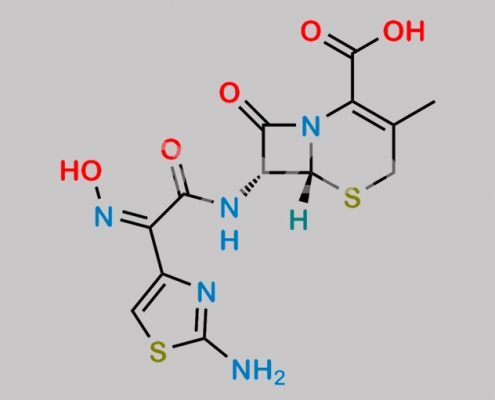
178949-03-6,Cefdinir
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-thiazolylacetyl-glycine-oxime-impurity-usp.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-06 22:56:47 2024-07-06 22:56:47 Cefdinir Thiazolylacetyl Glycine Oxime Impurity (USP) CAS号 178949-03-6 2024年7月6日
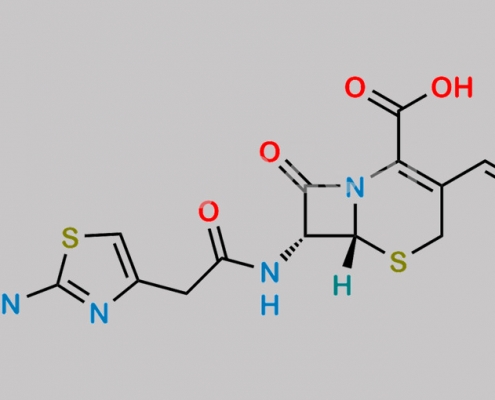
79350-10-0,Cefdinir
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-usp-rc-b.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-06 22:56:47 2024-07-06 22:56:47 Cefdinir USP Related Compound B CAS号 79350-10-0 2024年7月6日
71091-93-5,Cefdinir
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-3-methyl-impurity-usp.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-06 22:41:24 2024-07-06 22:41:24 Cefdinir 3-Methyl Impurity (USP) CAS号 71091-93-5 2024年7月6日
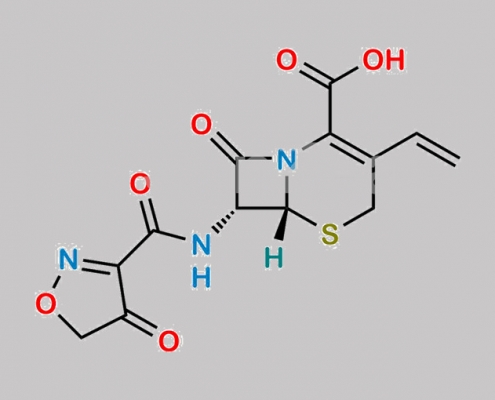
1356842-10-8,Cefdinir
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-isoxazole-analog-usp.jpg
510
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
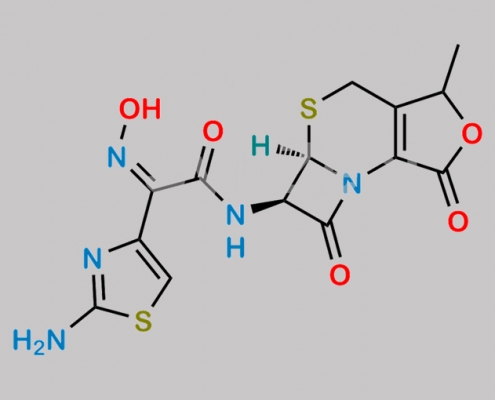
great_watson-int 2024-07-06 22:41:24 2024-07-06 22:41:24 Cefdinir Isoxazole Analog (USP) CAS号 1356842-10-8 2024年7月6日
178601-88-2,Cefdinir
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-e-isomer-usp.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int 2024-07-06 22:37:47 2024-07-06 22:37:47 Cefdinir (E)-Isomer (USP) CAS号 178601-88-2
Scroll to top
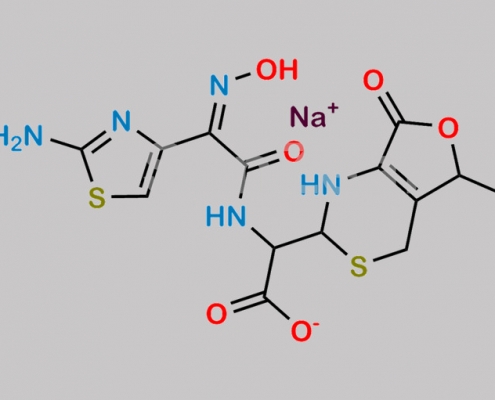 https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-usp-related-compound-asodium-salt.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
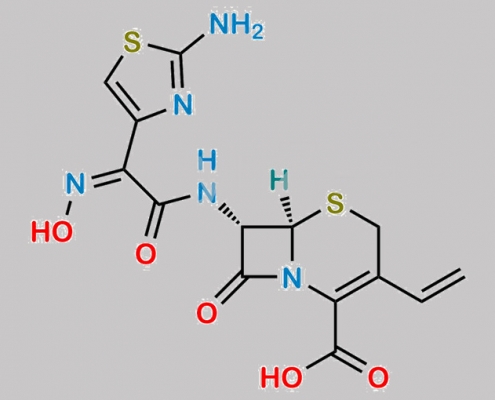
great_watson-int2024-07-06 22:58:552024-07-06 22:58:55Cefdinir USP Related Compound A (Sodium salt) CAS号 N/A
https://www.watson-int.cn/wp-content/uploads/2024/07/cefdinir-usp-related-compound-asodium-salt.jpg
511
1200
great_watson-int
https://www.watson-int.cn/wp-content/uploads/2019/10/new-logo-300x300.jpg
great_watson-int2024-07-06 22:58:552024-07-06 22:58:55Cefdinir USP Related Compound A (Sodium salt) CAS号 N/A