文章
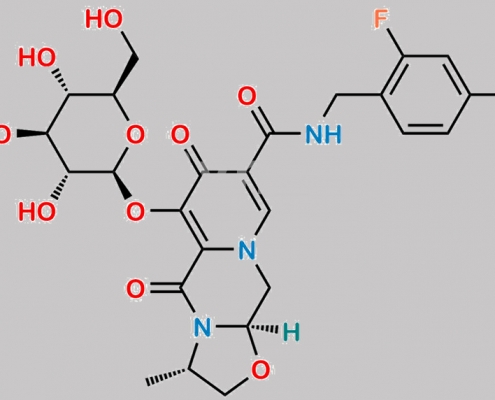
Cabotegravir O-glucuronide M2 CAS号 2170827-76-4
2170827-76-4,Cabotegravir
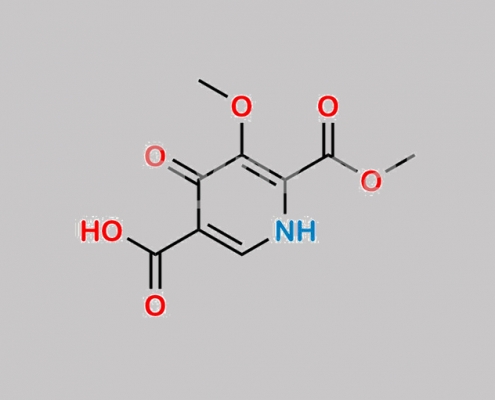
Cabotegravir Desalkyl 杂质 CAS号 N/A
N/A,Cabotegravir
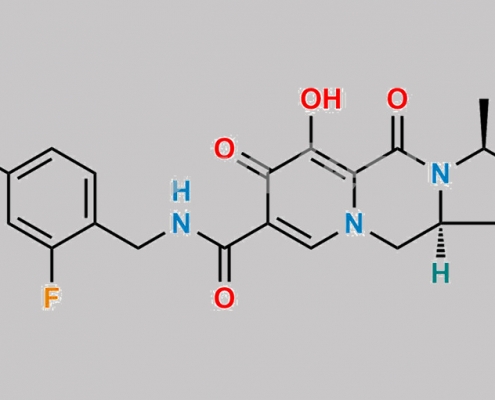
Cabotegravir Diastereomer-1 杂质 CAS号 1646862-08-9
1646862-08-9,Cabotegravir
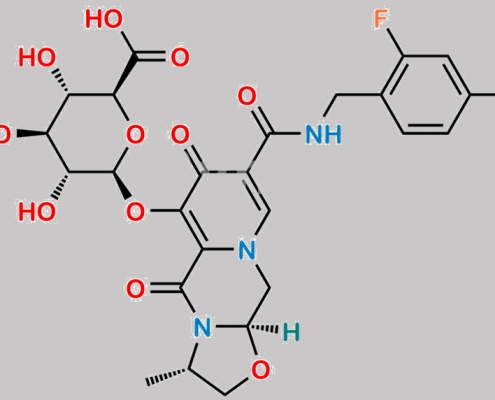
Cabotegravir O-glucuronide M1 CAS号 2170827-75-3
2170827-75-3,Cabotegravir
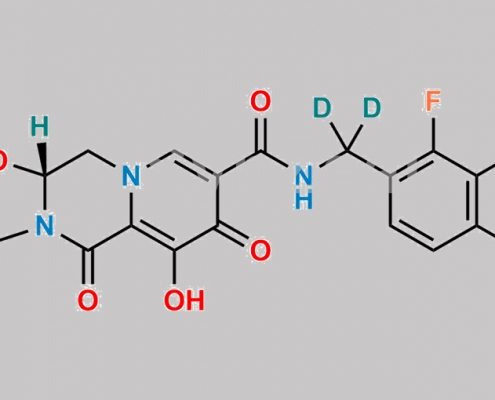
Cabotegravir D3 (Possibility 1) CAS号 N/A
N/A,Cabotegravir
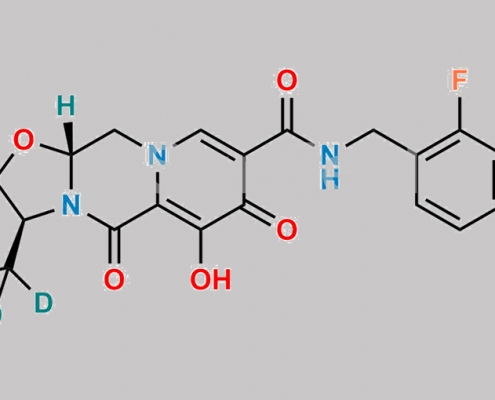
Cabotegravir-D5 CAS号 2750534-77-9
2750534-77-9,Cabotegravir
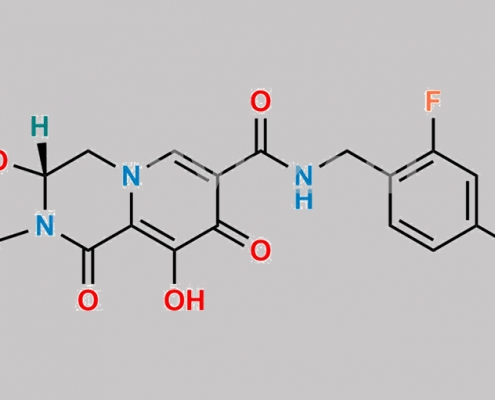
Cabotegravir CAS号 1051375-10-0
1051375-10-0,Cabotegravir
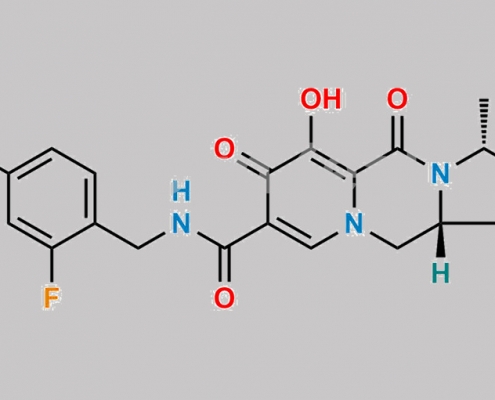
Cabotegravir Diastereomer-2 杂质 CAS号 N/A
N/A,Cabotegravir
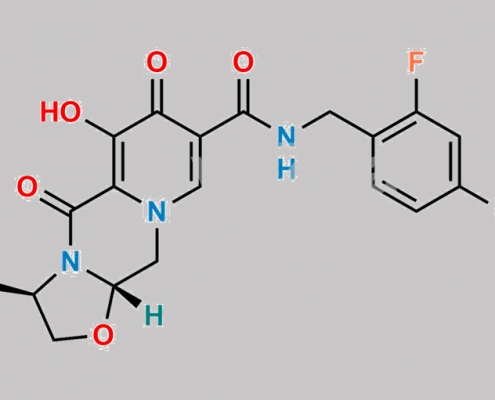
Cabotegravir Enantiomer 杂质 CAS号 1309560-64-2
1309560-64-2,Cabotegravir
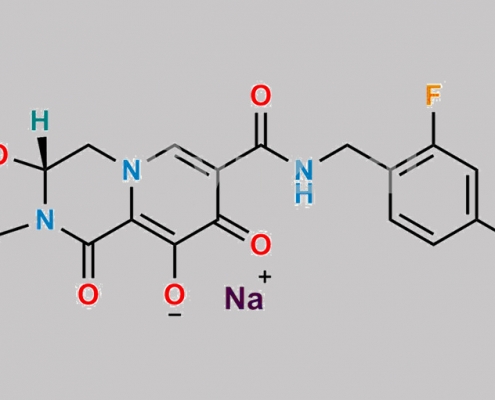
Cabotegravir Sodium CAS号 1051375-13-3
1051375-13-3,Cabotegravir





