文章
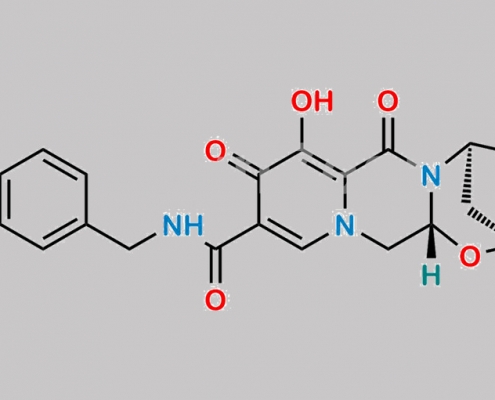
Bictegravir 4-Fluoro 杂质 CAS号 N/A
N/A,Bictegravir
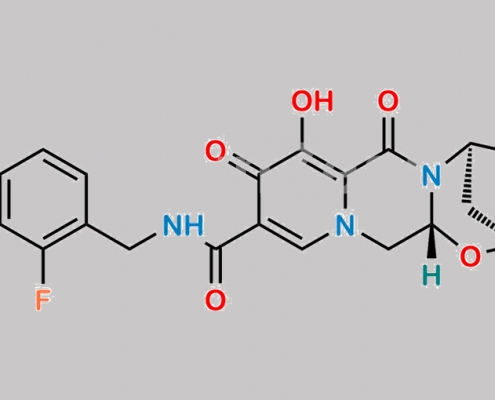
Bictegravir 2,4-Difluoro 杂质 CAS号 1611493-56-1
1611493-56-1,Bictegravir
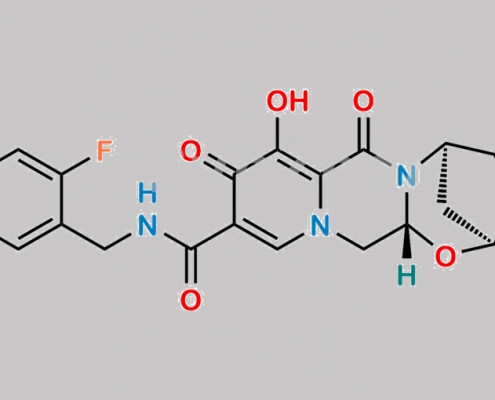
Bictegravir 2-Fluoro 杂质 CAS号 N/A
N/A,Bictegravir
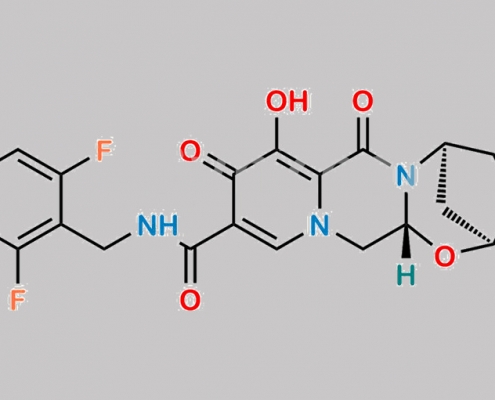
Bictegravir 2,6-Difluoro 杂质 CAS号 N/A
N/A,Bictegravir
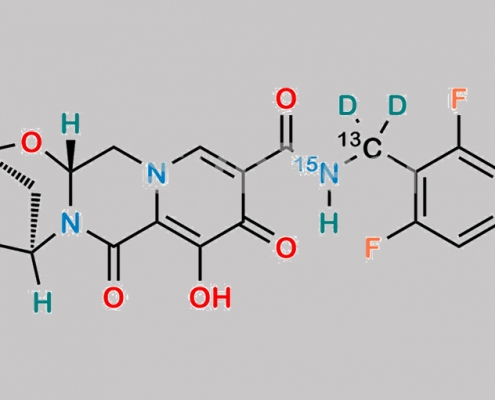
Bictegravir-13C-15N-d2 CAS号 N/A
N/A,Bictegravir

Bictegravir 杂质 4 CAS号 N/A
N/A,Bictegravir
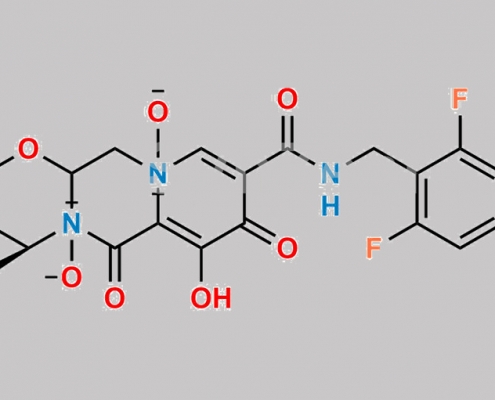
Bictegravir 杂质 5 CAS号 N/A
N/A,Bictegravir
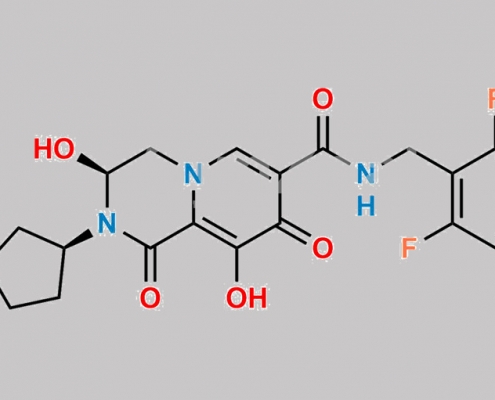
Bictegravir 杂质 3 CAS号 N/A
N/A,Bictegravir
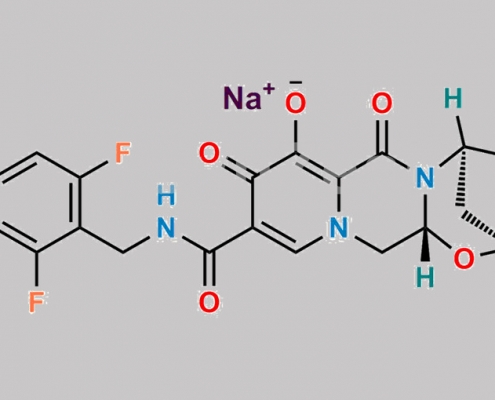
Bictegravir Sodium CAS号 1807988-02-8
1807988-02-8,Bictegravir
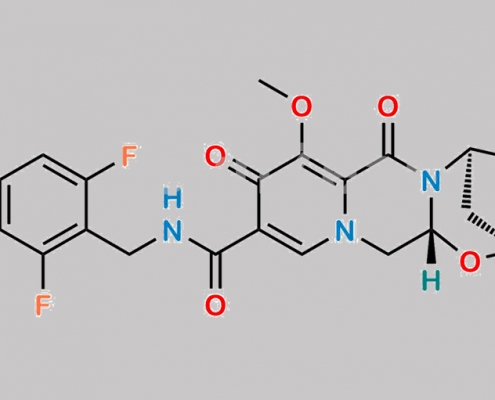
Methyl Bictegravir CAS号 1616340-94-3
1616340-94-3,Bictegravir





