文章
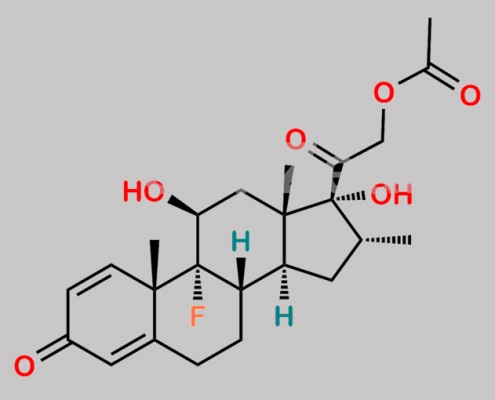
Betamethasone Acetate EP Impurity B CAS号 1177-87-3
1177-87-3,Betamethasone
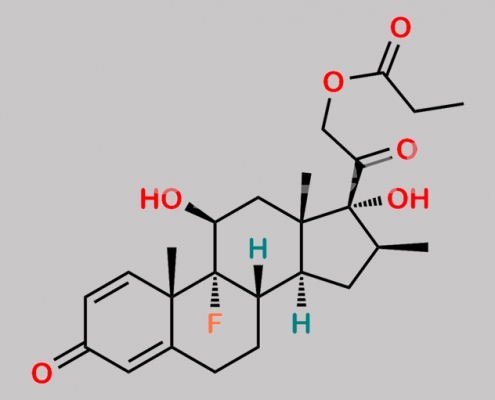
Betamethasone Dipropionate EP Impurity C CAS号 75883-07-7
75883-07-7,Betamethasone
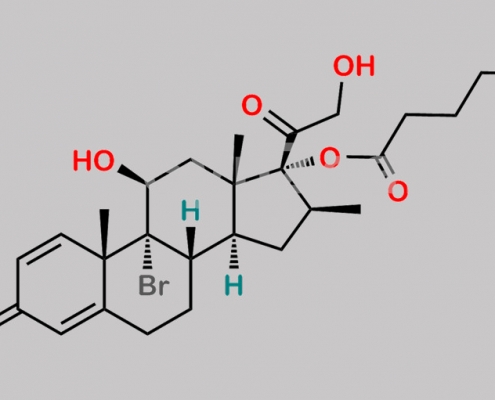
Betamethasone Valerate EP Impurity D CAS号 N/A
N/A,Betamethasone
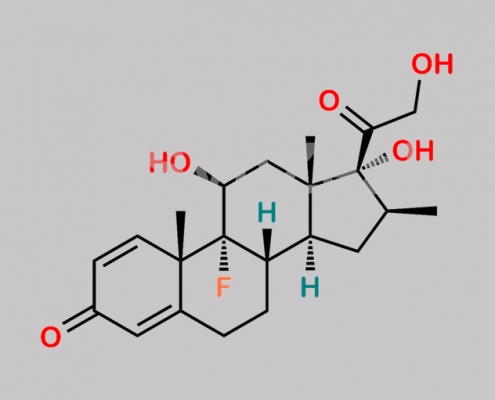
Betamethasone Acetate EP Impurity A CAS号 378-44-9
378-44-9,Betamethasone
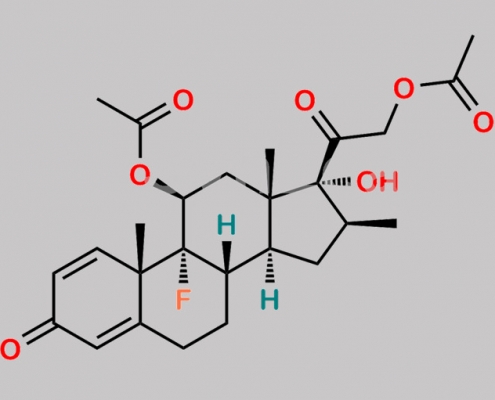
Betamethasone Acetate EP Impurity C CAS号 330157-05-6
330157-05-6,Betamethasone

Betamethasone CAS号 378-44-9
378-44-9,Betamethasone
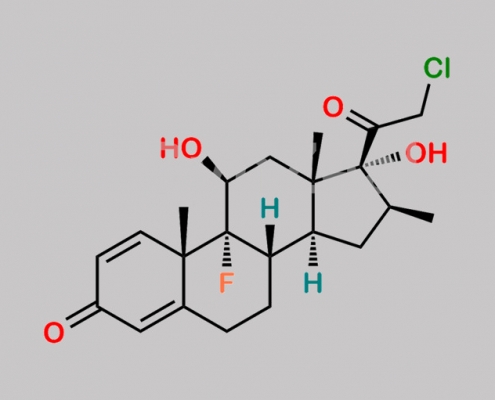
Betamethasone EP Impurity B CAS号 25122-41-2
25122-41-2,Betamethasone
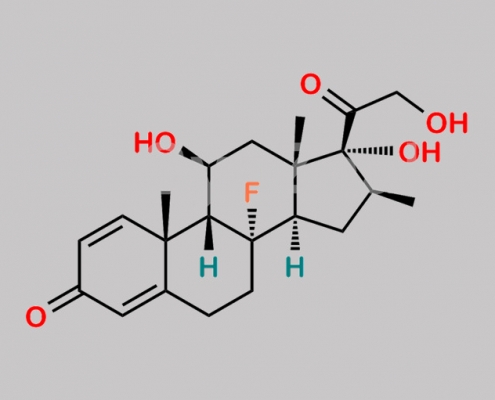
Betamethasone EP Impurity I CAS号 185613-69-8
185613-69-8,Betamethasone
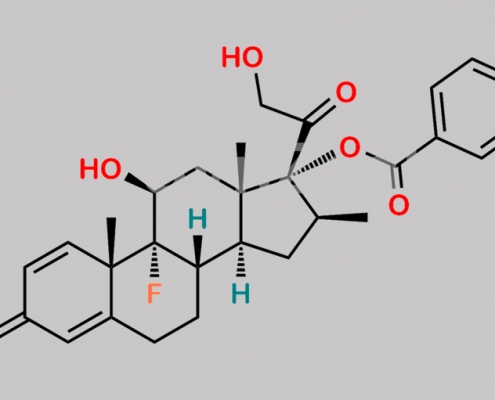
Betamethasone Benzoate CAS号 22298-29-9
22298-29-9,Betamethasone
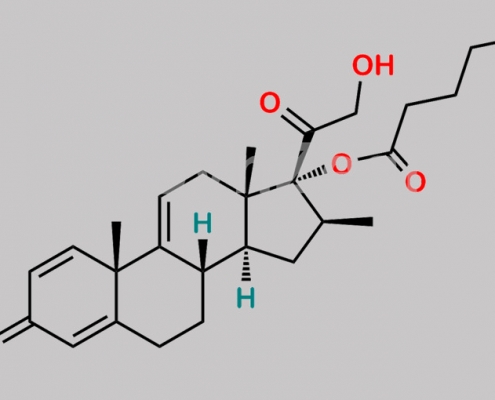
Betamethasone Valerate EP Impurity F CAS号 16125-28-3
16125-28-3,Betamethasone





