文章
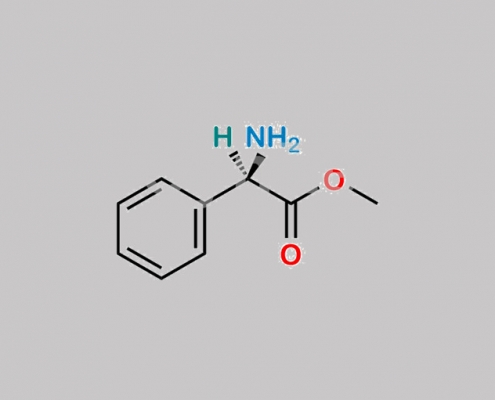
Bacampicillin EP 杂质 G CAS号 24461-61-8
24461-61-8,Bacampicillin
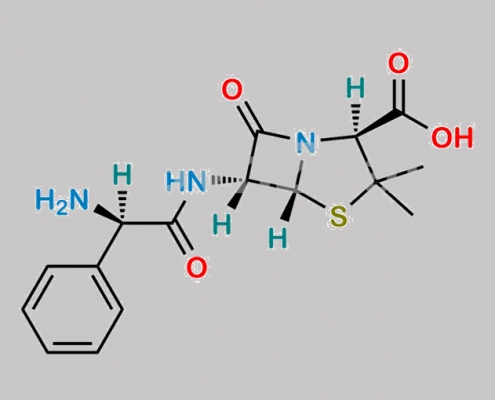
Bacampicillin EP 杂质 I CAS号 69-53-4
69-53-4,Bacampicillin
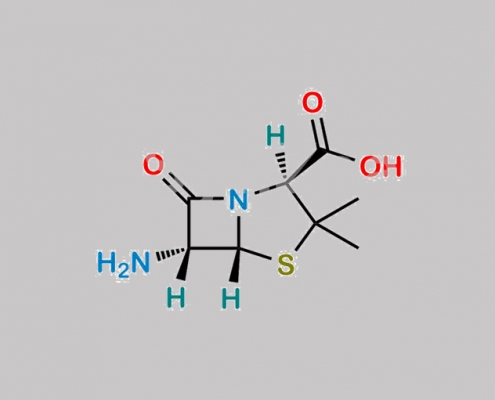
Bacampicillin EP 杂质 A CAS号 551-16-6
551-16-6,Bacampicillin
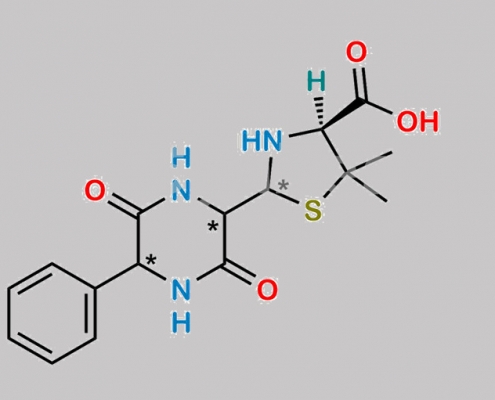
Bacampicillin EP 杂质 E CAS号 N/A
N/A,Bacampicillin
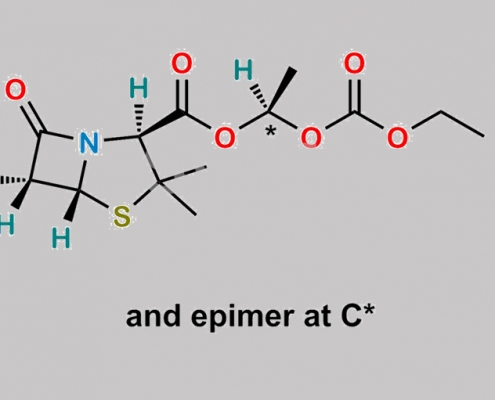
Bacampicillin Hydrochloride CAS号 37661-08-8
37661-08-8,Bacampicillin
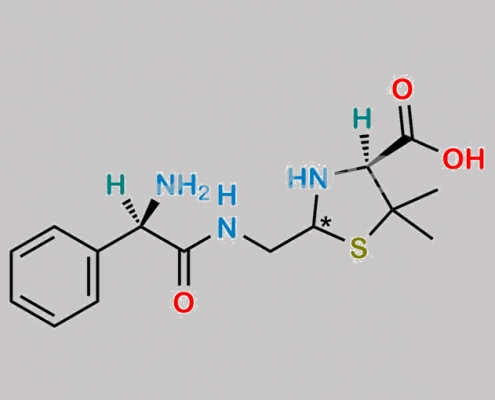
Bacampicillin EP 杂质 C CAS号 N/A
N/A,Bacampicillin
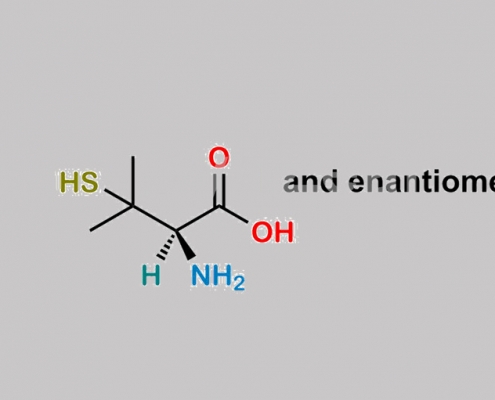
Bacampicillin EP 杂质 F CAS号 52-66-4
52-66-4,Bacampicillin
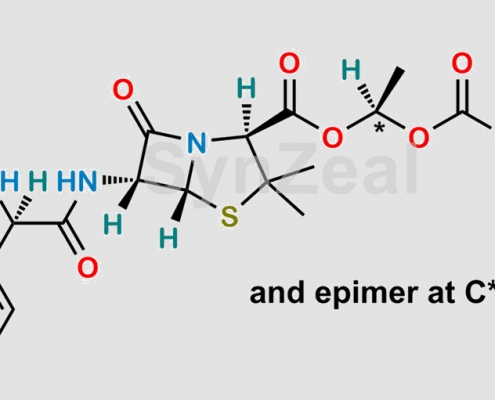
Bacampicillin EP 杂质 H CAS号 N/A
N/A,Bacampicillin
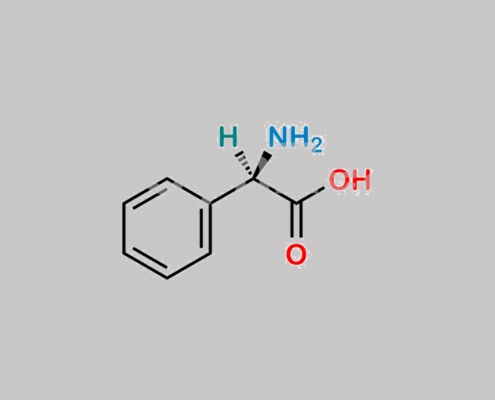
Bacampicillin EP 杂质 B CAS号 875-74-1
875-74-1,Bacampicillin
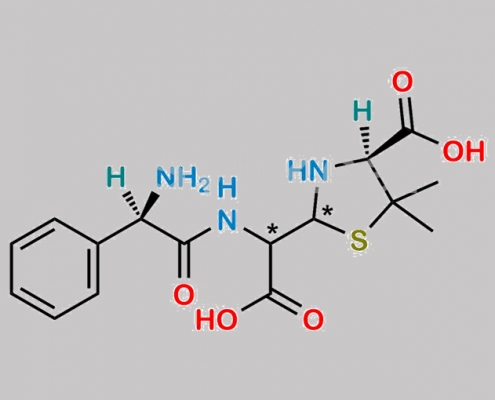
Bacampicillin EP 杂质 D CAS号 1642629-93-3
1642629-93-3,Bacampicillin





