Mometasone EP Impurity F CAS号 1305334-30-8
基本信息
CAS(化学文摘登记)号
1305334-30-8
英文名称
Mometasone EP Impurity F
英文化学名
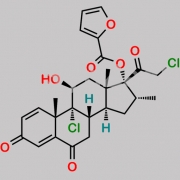
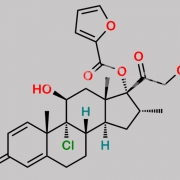
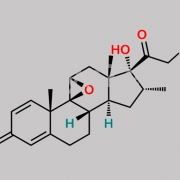
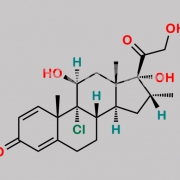
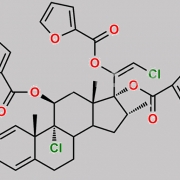
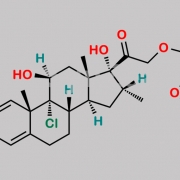
9,21-Dichloro-11beta-hydroxy-16alpha-methyl-3,6,20-trioxopregna-1,4-dien-17-yl furan-2-carboxylate (as per EP)
简化分子线性输入规范(SMILES)
O=C(C1=CC=CO1)O[C@]2(C(CCl)=O)[C@H](C)C[C@@]3([H])[C@]4([H])CC(C5=CC(C=C[C@]5(C)[C@@]4(Cl)[C@@H](O)C[C@]23C)=O)=O
分子式
C27H28CI2O7
分子量
535.4
物理性质
外观
安全信息
危险品运输编号
非危险品
德国水污染物质分类清单(WGK Germany)
3
详细规格
测试方法
核磁;质谱,液相色谱
保存条件
常温避光保存
产品描述
Mometasone EP Impurity F is chemically 9,21-Dichloro-11beta-hydroxy-16alpha-methyl-3,6,20-trioxopregna-1,4-dien-17-yl furan-2-carboxylate (as per EP). It is also known as 6-Oxo Mometasone Furoate. Mometasone EP Impurity F is supplied with detailed characterization data compliant with regulatory guideline. Mometasone EP Impurity F can be used for the analytical method development, method validation (AMV), Quality Controlled (QC) application for Abbreviated New Drug Application (ANDA) or during commercial production of Mometasone . The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ChemWhat products are for analytical purpose only and not for human use.
快速询价及关联信息
快速询价
品牌
ChemWhat官网链接地址
https://www.chemwhat.com/mometasone-ep-impurity-f-cas-1305334-30-8/