Fusidic Acid EP 杂质 C CAS号 N/A
基本信息
CAS(化学文摘登记)号
N/A
英文名称
Fusidic Acid EP 杂质 C
英文化学名
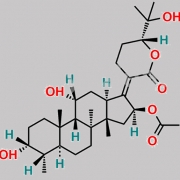
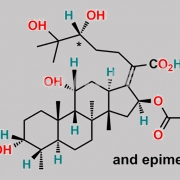
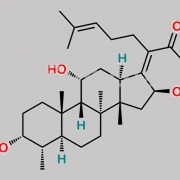
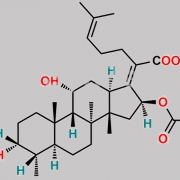
Ent-(17Z)-3beta,11beta-dihydroxy-17-[(6S)-6-(1-hydroxy-1-methylethyl)-2-oxodihydro-2H-pyran-3(4H)-ylidene]-4beta,8,14-trimethyl-18-nor-5beta,10alpha-androstan-16alpha-yl acetate (as per EP)
简化分子线性输入规范(SMILES)
C[C@@]1(C[C@]/2([H])OC(C)=O)[C@@]3(C)CC[C@@]4([H])[C@@](C)([H])[C@@](O)([H])CC[C@]4(C)[C@]3([H])[C@@](O)([H])C[C@@]1([H])C2=C5C(O[C@](C(O)(C)C)([H])CC5)=O
分子式
C31H48O7
分子量
532.7
物理性质
外观
安全信息
危险品运输编号
非危险品
德国水污染物质分类清单(WGK Germany)
3
详细规格
测试方法
核磁;质谱,液相色谱
保存条件
常温避光保存
产品描述
Fusidic Acid EP Impurity C is chemically Ent-(17Z)-3beta,11beta-dihydroxy-17-[(6S)-6-(1-hydroxy-1-methylethyl)-2-oxodihydro-2H-pyran-3(4H)-ylidene]-4beta,8,14-trimethyl-18-nor-5beta,10alpha-androstan-16alpha-yl acetate (as per EP). It is also known as (24R)-24,25-dihydro-24,25-dihydroxyfusidic acid 21,24-lactone (EP). Fusidic Acid EP Impurity C is supplied with detailed characterization data compliant with regulatory guideline. Fusidic Acid EP Impurity C can be used for the analytical method development, method validation (AMV), Quality Controlled (QC) application for Abbreviated New Drug Application (ANDA) or during commercial production of Fusidic Acid. The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ChemWhat products are for analytical purpose only and not for human use.
快速询价及关联信息
快速询价
品牌
ChemWhat官网链接地址
https://www.chemwhat.com/fusidic-acid-ep-impurity-c-cas-N/A/