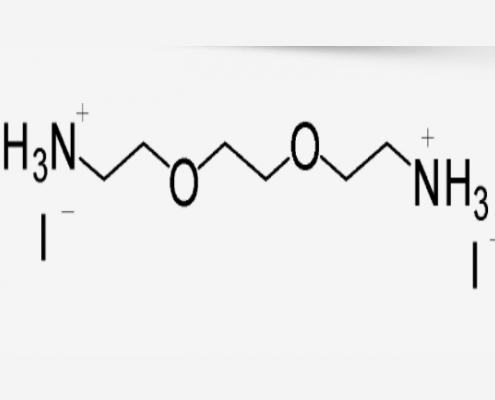
2,2’-(乙烯二氧)双乙胺氢碘酸盐(2,2’-(乙烯二氧)双乙胺碘) (C6H18I2N2O2) CAS 2044283-93-2
2,2’-(乙烯二氧)双乙胺氢碘酸盐(2,2’-(乙烯二氧)双乙胺碘)(C6H18I2N2O2),CAS 2044283-93-2
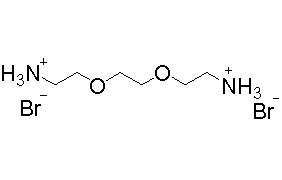
2,2’-(乙烯二氧)双乙胺氢溴酸盐(2,2’-(乙烯二氧)双乙胺溴) (C6H18Br2N2O2) CAS 80685-25-20
2,2’-(乙烯二氧)双乙胺氢溴酸盐(2,2’-(乙烯二氧)双乙胺溴)(C6H18Br2N2O2),CAS 80685-25-20
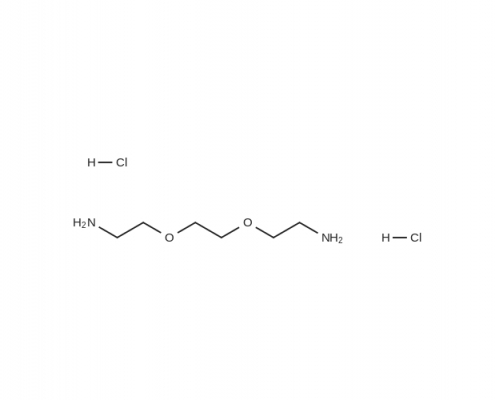
2,2’-(乙烯二氧)双乙胺盐酸盐(2,2’-(乙烯二氧)双乙胺氯) (C6H18Cl2N2O2) CAS 80685-25-2
2,2’-(乙烯二氧)双乙胺盐酸盐(2,2’-(乙烯二氧)双乙胺氯)(C6H18Cl2N2O2),CAS 80685-25-2
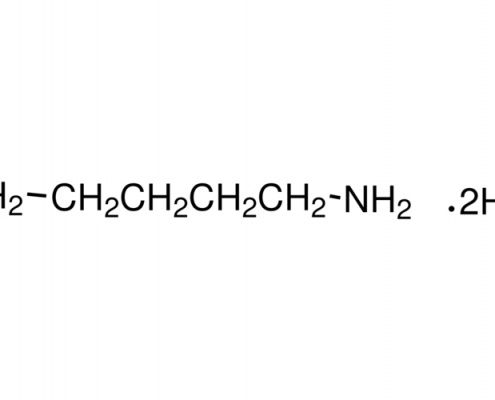
1,4-丁二胺盐酸盐(丁二胺氯) (C4H14Cl2N2) CAS 333-93-7
1,4-丁二胺盐酸盐(丁二胺氯)(C4H14Cl2N2),CAS 333-93-7
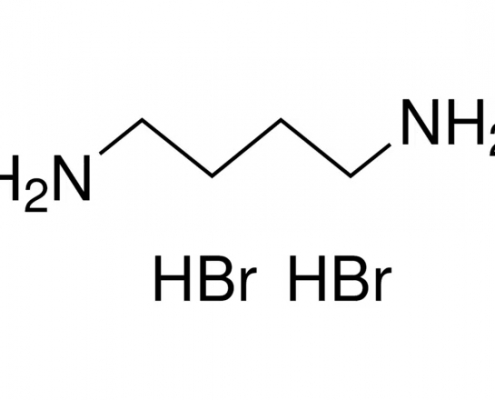
1,4-丁二胺氢溴酸盐(丁二胺溴) (C4H14Br2N2) CAS 18773-04-1
1,4-丁二胺氢溴酸盐(丁二胺溴)(C4H14Br2N2),CAS 18773-04-1
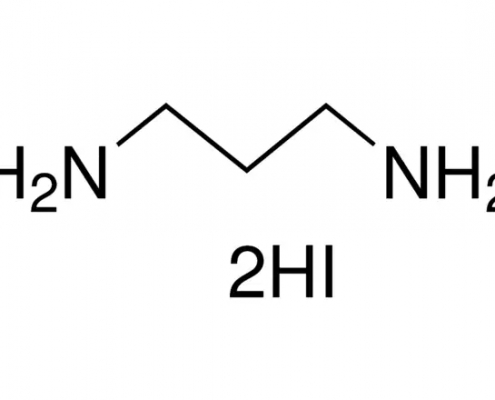
1,3-丙二胺氢碘酸盐(丙二胺碘) (C3H12I2N2) CAS 120675-53-8
1,3-丙二胺氢碘酸盐(丙二胺碘)(C3H12I2N2),CAS 120675-53-8
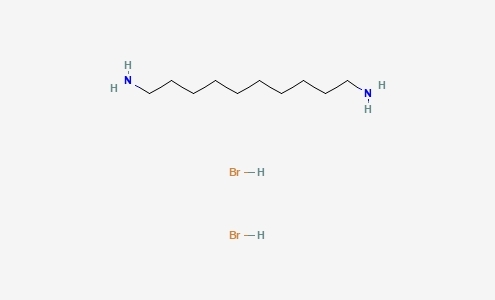
1,10-癸二胺氢溴酸盐(癸二胺溴) (C10H26Br2N2) CAS 473402-65-2
1,10-癸二胺氢溴酸盐(癸二胺溴)(C10H26Br2N2),CAS 473402-65-2
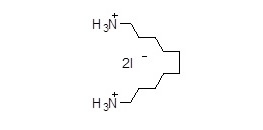
1,10-癸二胺氢碘酸盐(癸二胺碘) (C10H26I2N2) CAS 473402-65-20
1,10-癸二胺氢碘酸盐(癸二胺碘)(C10H26I2N2),CAS 473402-65-20
二甲胺氢溴酸盐(二甲胺溴) (C2H8BrN) CAS 6912/12/5
二甲胺氢溴酸盐(二甲胺溴)(C2H8BrN),CAS 6912/12/5
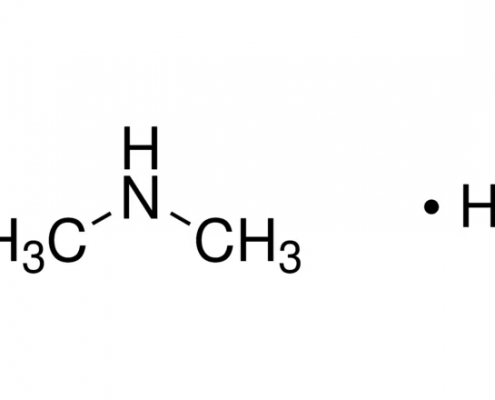
二甲胺氢碘酸盐(二甲胺碘) (C2H8IN) CAS 51066-74-1
二甲胺氢碘酸盐(二甲胺碘)(C2H8IN),CAS 51066-74-1





